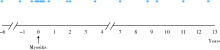北京大学学报(医学版) ›› 2020, Vol. 52 ›› Issue (6): 995-1000. doi: 10.19723/j.issn.1671-167X.2020.06.002
肌炎合并血栓栓塞患者的临床及免疫学特征
朱冯赟智1,邢晓燕2,汤晓菲3,李依敏1,邵苗1,张学武1,李玉慧1,△( ),孙晓麟1,何菁1
),孙晓麟1,何菁1
- 1. 北京大学人民医院风湿免疫科,北京 100044
2. 北京大学人民医院心脏电生理室,北京 100044
3. 航天中心医院肾内风湿科,北京 100069
Clinical and immunological characteristics of myositis complicated with thromboembolism
Feng-yun-zhi ZHU1,Xiao-yan XING2,Xiao-fei TANG3,Yi-min LI1,Miao SHAO1,Xue-Wu ZHANG1,Yu-hui LI1,△( ),Xiao-lin SUN1,Jing HE1
),Xiao-lin SUN1,Jing HE1
- 1. Department of Rheumatology and Immunology, Peking University People’s Hospital, Beijing 100044, China
2. Department of Cardiac Electrophysiology, Peking University People’s Hospital, Beijing 100044, China
3. Department of Nephrology and Rheumatology, Aerospace Center Hospital, Beijing 100069, China
摘要:
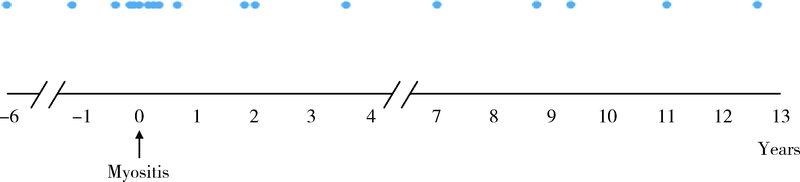
目的:探讨合并血栓栓塞的肌炎患者的临床及免疫学特征。方法:回顾性分析2003—2019年于北京大学人民医院住院的390例肌炎患者的病历资料,包括人口学特征、皮肤、肌肉表现、脏器受累、实验室指标(肌酶、白蛋白、凝血功能、炎性指标、肌炎特异性/相关性抗体)、治疗方案,并根据有无合并血栓栓塞将患者进行分组比较,应用Logistic回归分析明确肌炎合并血栓栓塞的危险因素。结果:肌炎患者平均起病年龄(49.6±13.4)岁,男女比例为0.31:1,血栓栓塞发生率为4.62%(18/390),其中,55.6%(10/18)为下肢深静脉血栓形成,其次为脑梗塞(22.2%,4/18)、肺栓塞(11.1%,2/18)、肾动脉栓塞(5.6%,1/18)、上肢静脉血栓形成(5.6 %,1/18)。血栓栓塞发生时间方面,38.9%(7/18)发生在肌炎诊断的前后6个月内,50%(9/18)发生在肌炎确诊后。合并血栓栓塞的肌炎患者起病年龄偏大,平均(58.3±11.7)岁,合并糖尿病(44.4% vs. 16.4%,P=0.006)、冠心病(22.2% vs. 3.0%,P=0.003)、 90 d内手术史(16.7% vs. 3.5%,P=0.032)等并发症均高于无血栓栓塞组。实验室检查方面,合并血栓栓塞的肌炎患者C-反应蛋白(12.2 mg/L vs. 4.1 mg/L,P<0.001)、血清铁蛋白(20 085.5 μg/L vs. 216.6 μg/L,P<0.001)、血清白蛋白水平(32.4 g/L vs. 36.5 g/L,P=0.002)、D-二聚体(529.0 μg/L vs. 268.0 μg/L,P=0.002)均明显升高,活化部分凝血酶原时间(26.9 s vs. 28.7 s,P=0.049)显著低于无血栓栓塞组。肌炎发生血栓栓塞的危险因素包括:白蛋白水平降低(OR=0.831,95%CI:0.736~0.939,P=0.003)、合并糖尿病(OR=4.468,95%CI:1.382~14.448,P=0.012)、合并冠心病(OR=22.079,95%CI:3.589~135.837,P=0.001)。未见肌炎特异性抗体与血栓栓塞发生的相关性。结论:肌炎患者可发生血栓栓塞事件,合并白蛋白水平降低、糖尿病、冠心病的肌炎患者发生血栓栓塞的风险高,临床应高度警惕。
中图分类号:
- R593.2
| [1] |
Lundberg IE, Tjärnlund A, Bottai M, et al. 2017 European League Against Rheumatism/American College of rheumatology classification criteria for adult and juvenile idiopathic inflammatory myopathies and their major subgroups[J]. Arthritis Rheumatol, 2017,69(12):2271-2282.
doi: 10.1002/art.40320 pmid: 29106061 |
| [2] |
Ogdie A, Kay McGill N, Shin DB, et al. Risk of venous thromboembolism in patients with psoriatic arthritis, psoriasis and rheumatoid arthritis: A general population-based cohort study[J]. Eur Heart J, 2018,39(39):3608-3614.
doi: 10.1093/eurheartj/ehx145 pmid: 28444172 |
| [3] |
Aviña-Zubieta JA, Vostretsova K, De Vera MA, et al. The risk of pulmonary embolism and deep venous thrombosis in systemic lupus erythematosus: A general population-based study[J]. Semin Arthritis Rheum, 2015,45(2):195-201.
doi: 10.1016/j.semarthrit.2015.05.008 pmid: 26364556 |
| [4] |
Carruthers EC, Choi HK, Sayre EC, et al. Risk of deep venous thrombosis and pulmonary embolism in individuals with polymyositis and dermatomyositis: A general population-based study[J]. Ann Rheum Dis, 2016,75(1):110-116.
doi: 10.1136/annrheumdis-2014-205800 pmid: 25193998 |
| [5] | Antovic A, Notarnicola A, Svensson J, et al. Venous thromboembolic events in idiopathic inflammatory myopathy: Occurrence and relation to disease onset[J]. Arthritis Care Res (Hoboken), 2018,70(12):1849-1855. |
| [6] | Bohan A, Peter J B. Polymyositis and dermatomyositis (first of two parts)[J]. N Engl J Med, 1975,292(7):403-407. |
| [7] |
Sontheimer RD. Would a new name hasten the acceptance of amyopathic dermatomyositis (dermatomyositis siné myositis) as a distinctive subset within the idiopathic inflammatory dermatomyo-pathies spectrum of clinical illness?[J]. J Am Acad Dermatol, 2002,46(4):626-636.
pmid: 11907524 |
| [8] |
Smith SC Jr, Allen J, Blair SN, et al. AHA/ACC guidelines for secondary prevention for patients with coronary and other atherosclerotic vascular disease: 2006 update: endorsed by the National Heart, Lung, and Blood Institute[J]. Circulation, 2006,113(19):2363-2372.
pmid: 16702489 |
| [9] |
Heit JA, Spencer FA, White RH. The epidemiology of venous thromboembolism[J]. J Thromb Thrombolysis, 2016,41(1):3-14.
pmid: 26780736 |
| [10] |
Holmqvist M, Ljung L, Askling J. Acute coronary syndrome in new-onset rheumatoid arthritis: A population-based nationwide cohort study of time trends in risks and excess risks[J]. Ann Rheum Dis, 2017,76(10):1642-1647.
doi: 10.1136/annrheumdis-2016-211066 pmid: 28710095 |
| [11] | Schoenfeld SR, Choi HK, Sayre EC, et al. Risk of pulmonary embolism and deep venous thrombosis in systemic sclerosis: A general population-based study[J]. Arthritis Care Res (Hoboken), 2016,68(2):246-253. |
| [12] | Zöller B, Li X, Sundquist J, et al. Risk of pulmonary embolism in patients with autoimmune disorders: A nationwide follow-up study from Sweden[J]. Lancet, 2012,379(9812):244-249. |
| [13] |
Xu J, Lupu F, Esmon CT. Inflammation, innate immunity and blood coagulation[J]. Hamostaseologie, 2010,30(1):5-9.
pmid: 20162248 |
| [14] |
Choi HK, Rho YH, Zhu Y, et al. The risk of pulmonary embo-lism and deep vein thrombosis in rheumatoid arthritis: A UK population-based outpatient cohort study[J]. Ann Rheum Dis, 2013,72(7):1182-1187.
pmid: 22930596 |
| [15] |
Saghazadeh A, Rezaei N. Inflammation as a cause of venous thromboembolism[J]. Crit Rev Oncol Hematol, 2016,99:272-285.
doi: 10.1016/j.critrevonc.2016.01.007 pmid: 26811138 |
| [16] | Mussbacher M, Salzmann M, Brostjan C, et al. Cell type-specific roles of NF-κB linking inflammation and thrombosis [J/OL]. Front Immunol, (2019-02-04)[2020-04-30]. doi: 10.3389/fimmu.2019.00085. |
| [17] | Ramagopalan SV, Wotton CJ, Handel AE, et al. Risk of venous thromboembolism in people admitted to hospital with selected immune-mediated diseases: Record-linkage study [J/OL]. BMC Med, (2011-01-10)[2020-04-30]. doi: 10.1186/1741-7015-9-1. |
| [1] | 肖云抒,朱冯赟智,罗澜,邢晓燕,李玉慧,张学武,沈丹华. 88例重叠肌炎的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53(6): 1088-1093. |
| [2] | 罗澜,邢晓燕,肖云抒,陈珂彦,朱冯赟智,张学武,李玉慧. 抗合成酶综合征合并心脏受累患者的临床及免疫学特征[J]. 北京大学学报(医学版), 2021, 53(6): 1078-1082. |
| [3] | 张朴丽,杨红霞,张立宁,葛勇鹏,彭清林,王国春,卢昕. 血清YKL-40在诊断抗黑色素瘤分化相关基因5阳性皮肌炎合并严重肺损伤中的价值[J]. 北京大学学报(医学版), 2021, 53(6): 1055-1060. |
| [4] | 伊文霞,魏翠洁,吴晔,包新华,熊晖,常杏芝. 长疗程利妥昔单抗治疗难治性幼年型特发性炎症性肌病3例[J]. 北京大学学报(医学版), 2021, 53(6): 1191-1195. |
| [5] | 吴燕芳,高飞,林滇恬,陈志涵,林禾. 托法替布联合治疗抗MDA5抗体阳性的无肌病皮肌炎并发快速进展型间质性肺病5例临床分析[J]. 北京大学学报(医学版), 2021, 53(5): 1012-1016. |
| [6] | 郑艺明,郝洪军,刘怡琳,郭晶,赵亚雯,张巍,袁云. Ro52抗体与其他肌炎抗体共阳性的相关性研究[J]. 北京大学学报(医学版), 2020, 52(6): 1088-1092. |
| [7] | 甘雨舟,李玉慧,张丽华,马琳,何文雯,金月波,安媛,栗占国,叶华. 临床无肌病性皮肌炎与皮肌炎临床及免疫学特征比较[J]. 北京大学学报(医学版), 2020, 52(6): 1001-1008. |
| [8] | 赵静,孙峰,李云,赵晓珍,徐丹,李英妮,李玉慧,孙晓麟. 抗α-1C微管蛋白抗体在系统性硬化症中的表达及临床意义[J]. 北京大学学报(医学版), 2020, 52(6): 1009-1013. |
| [9] | 杨红霞,田小兰,江薇,李文丽,刘青艳,彭清林,王国春,卢昕. 免疫介导坏死性肌病的临床和病理特征分析[J]. 北京大学学报(医学版), 2019, 51(6): 989-995. |
| [10] | 李英妮,相晓红,赵静,李云,孙峰,王红彦,贾汝琳,胡凡磊. 抗类瓜氨酸化抗体在系统性红斑狼疮中的意义[J]. 北京大学学报(医学版), 2019, 51(6): 1019-1024. |
| [11] | 徐婧,徐静,李鹤,唐杰,舒建龙,张婧,石连杰,李胜光. 皮肌炎合并IgA血管炎1例[J]. 北京大学学报(医学版), 2019, 51(6): 1173-1177. |
| [12] | 张志刚,刘新民. 急性肾梗死的临床特征:单中心52例临床分析[J]. 北京大学学报(医学版), 2019, 51(5): 863-869. |
| [13] | 张玮,张培训. 老年髋部骨折患者围手术期血栓预防时限分析[J]. 北京大学学报(医学版), 2019, 51(3): 501-504. |
| [14] | 杨伊莹,左晓霞,朱红林,刘思佳. 皮肌炎/多肌炎表观遗传学标志物的研究进展[J]. 北京大学学报(医学版), 2019, 51(2): 374-377. |
| [15] | 王永福,刘媛. 自身抗体在肿瘤及感染性疾病发生、发展中的作用[J]. 北京大学学报(医学版), 2018, 50(6): 952-955. |
|