北京大学学报(医学版) ›› 2021, Vol. 53 ›› Issue (6): 1115-1121. doi: 10.19723/j.issn.1671-167X.2021.06.018
有幽门螺杆菌感染家族史儿童胃部菌群的特点
- 北京大学第三医院儿科, 北京 100191
Characteristics of gastric microbiota in children with Helicobacter pylori infection family history
- Department of Pediatric, Peking University Third Hospital, Beijing 100191, China
摘要:
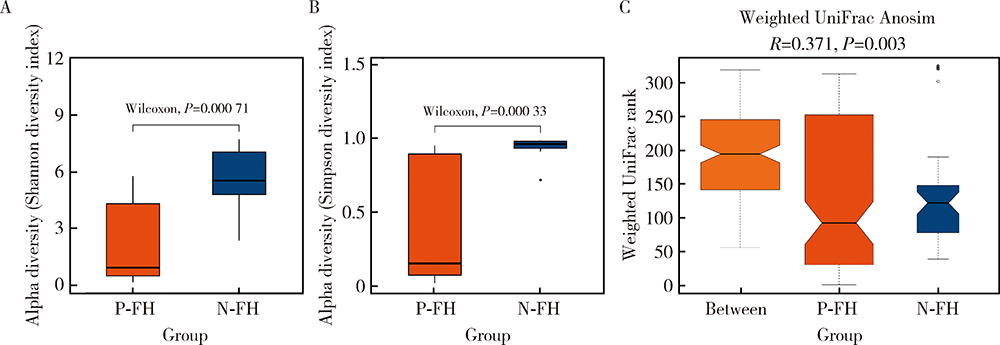
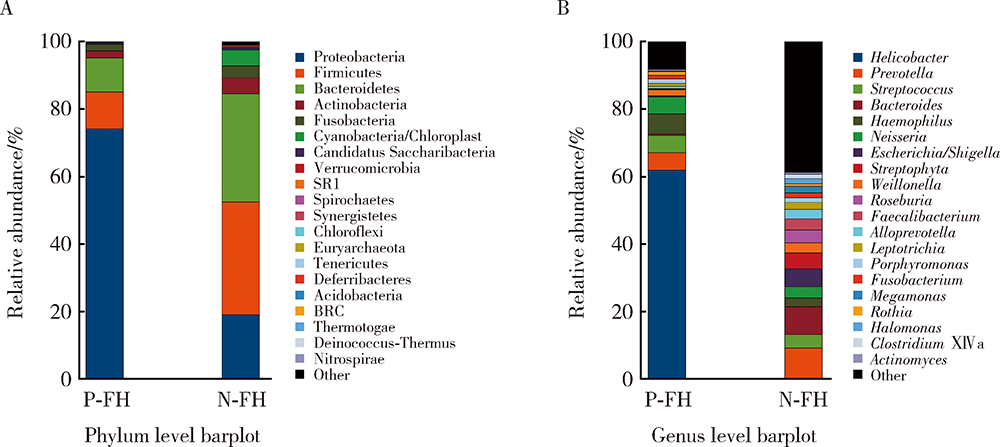
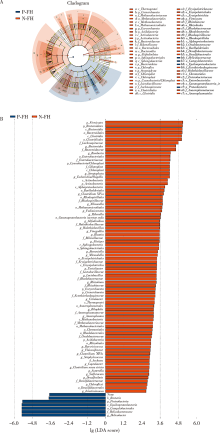
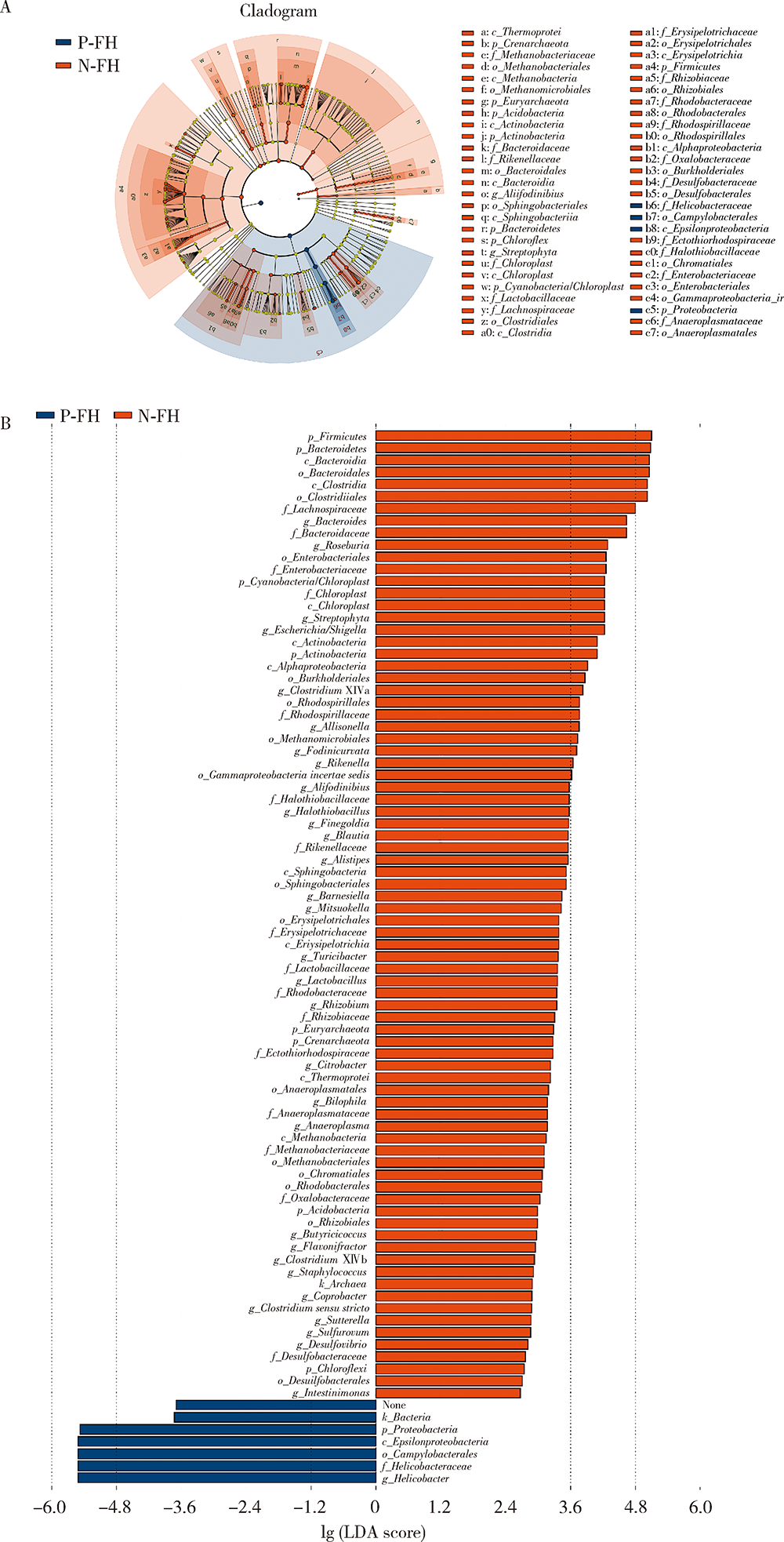
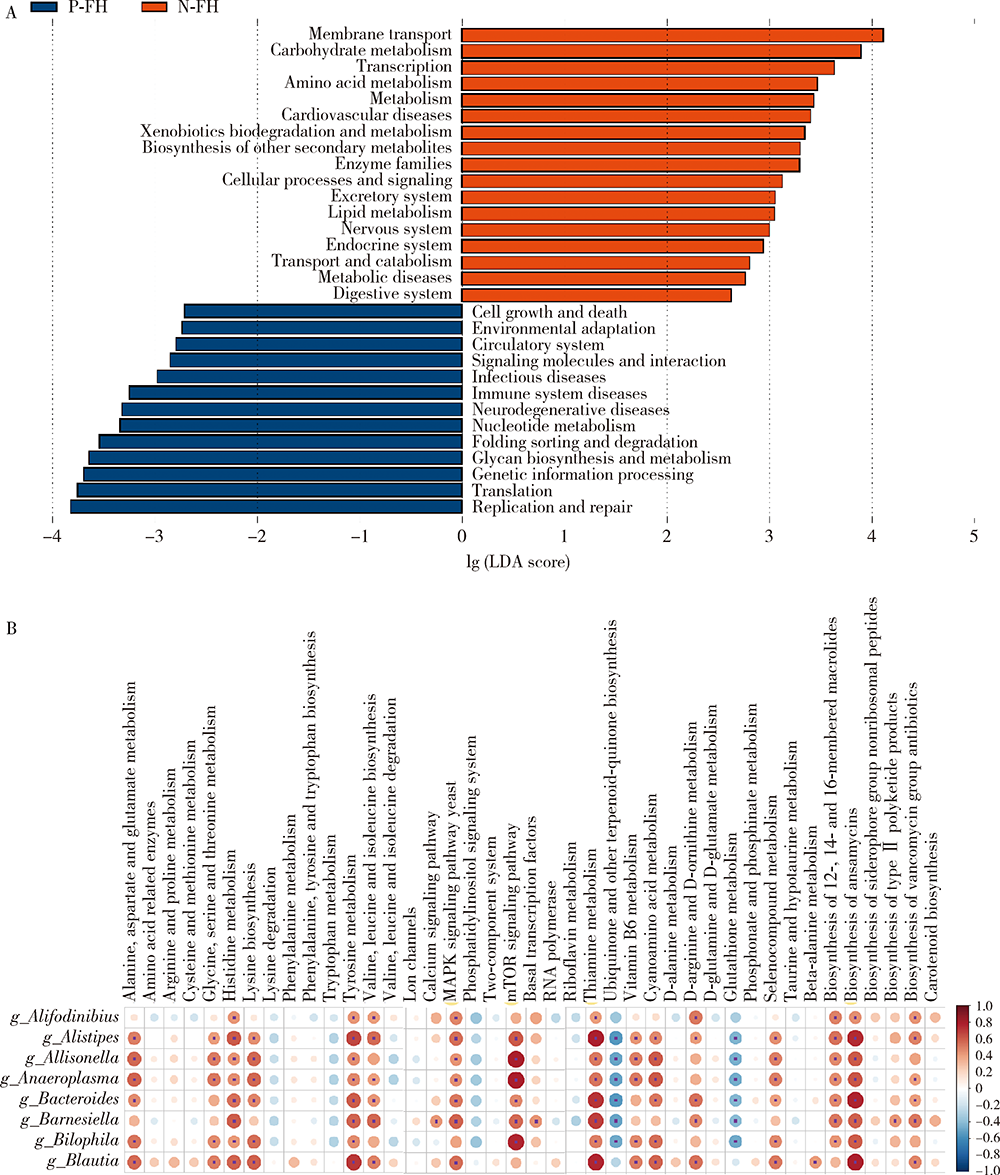
目的:探究有幽门螺杆菌(Helicobacter pylori, H. pylori)感染家族史,发生和未发生H. pylori感染的儿童胃部菌群特点。方法:分别采集患儿胃体和胃窦的黏膜标本,通过标本DNA提取、16S核糖体DNA(ribosomal DNA,rDNA)V3-V4区域PCR扩增、高通量测序、数据处理等步骤后,得到胃部黏膜菌群分析结果,将结果中有H. pylori感染家族史的标本根据是否发生H. pylori感染分为感染组(n=18)和非感染组(n=24),比较两组间菌群的α和β多样性、菌群丰度变化等指标,找出差异菌群,并对菌群功能进行预测分析。结果:感染组与非感染组胃部菌群α多样性和β多样性之间差异有统计学意义(P<0.05), 感染组的菌群多样性要低于非感染组。菌群相对丰度方面,门水平占优势的主要有变形菌门(Proteobacteria)、厚壁菌门(Firmicutes)、拟杆菌门(Bacteroidetes)、放线菌门(Actinobacteria)和梭杆菌门(Fusobacteria);属水平上,非感染组中拟杆菌(Bacteroides)、普雷沃氏菌(Prevotella)、链球菌(Streptococcus)和奈瑟菌(Neisseria)为优势菌种。差异物种方面,通过LEfSe分析,发现非感染组中属水平的拟杆菌属等的相对丰度显著高于感染组。功能预测发现,拟杆菌属与一些氨基酸和维生素代谢、丝裂原活化蛋白激酶(mitogen-activated protein kinase,MAPK)、哺乳动物雷帕霉素靶蛋白(mammalian target of rapamycin, mTOR)信号通路、安沙霉素(ansamycin)的合成相关通路均呈显著正相关。结论:有H. pylori感染家族史的儿童中,发生H. pylori感染和未发生感染者的胃部菌群存在显著差异,拟杆菌可能与儿童是否发生H. pylori感染存在关联。
中图分类号:
- R725.7
| [1] | Misak Z, Hojsak I, Homan M. Review: Helicobacter pylori in pediatrics[J]. Helicobacter, 2019, 24(Suppl 1):e12639. |
| [2] |
Weyermann M, Rothenbacher D, Brenner H. Acquisition of Helicobacter pylori infection in early childhood: Independent contributions of infected mothers, fathers, and siblings[J]. Am J Gastroenterol, 2009, 104(1):182-189.
doi: 10.1038/ajg.2008.61 pmid: 19098867 |
| [3] |
Ueno T, Suzuki H, Hirose M, et al. Influence of living environment during childhood on Helicobacter pylori infection in Japanese young adults[J]. Digestion, 2020, 101(6):779-784.
doi: 10.1159/000502574 |
| [4] |
Polk DB, Peek RM. Helicobacter Pylori: Gastric cancer and beyond[J]. Nat Rev Cancer, 2010, 10(6):403-414.
doi: 10.1038/nrc2857 |
| [5] |
Waskito LA, Salama NR, Yamaoka Y. Pathogenesis of Helicobac-ter pylori infection[J]. Helicobacter, 2018, 23(Suppl 1):e12516.
doi: 10.1111/hel.2018.23.issue-S1 |
| [6] |
Yan R, Guo Y, Gong Q, et al. Microbiological evidences for gastric cardiac microflora dysbiosis inducing the progression of inflammation[J]. J Gastroenterol Hepatol, 2020, 35(6):1032-1041.
doi: 10.1111/jgh.v35.6 |
| [7] | 中华医学会儿科学分会消化学组, 《中华儿科杂志》编辑委员会. 儿童幽门螺杆菌感染诊治专家共识[J]. 中华儿科杂志, 2015, 53(7):496-498. |
| [8] |
Llorca L, Pérez-Pérez G, Urruzuno P, et al. Characterization of the gastric microbiota in a pediatric population according to Helicobacter pylori status[J]. Pediatr Infect Dis J, 2017, 36(2):173-178.
doi: 10.1097/INF.0000000000001383 pmid: 27820723 |
| [9] | 彭贤慧, 周丽雅, 何利华, 等. 幽门螺杆菌感染者胃内菌群特征分析[J]. 胃肠病学和肝病学杂志, 2017, 26(6):658-663. |
| [10] |
Bik EM, Eckburg PB, Gill SR, et al. Molecular analysis of the bacterial microbiota in the human stomach[J]. Proc Natl Acad Sci U S A, 2006, 103(3):732-737.
doi: 10.1073/pnas.0506655103 |
| [11] | Bruno G, Rocco G, Zaccari P, et al. Helicobacter pylori infection and gastric dysbiosis: Can probiotics administration be useful to treat this condition?[J]. Can J Infect Dis Med Microbiol, 2018, 2018:6237239. |
| [12] | Delgado S, Leite AM, Ruas-Madiedo P, et al. Probiotic and technological properties of Lactobacillus spp. strains from the human stomach in the search for potential candidates against gastric microbial dysbiosis[J]. Front Microbiol, 2015, 5:766. |
| [13] |
Ascencio F, Gama NL, Philippis RD, et al. Effectiveness of Cyanothece spp. and Cyanospira capsulata exocellular polysaccharides as antiadhesive agents for blocking attachment of Helicobacter pylori to human gastric cells[J]. Folia Microbiol (Praha), 2004, 49(1):64-70.
doi: 10.1007/BF02931648 |
| [14] | Lorca GL, Wadstrom T, Valdez GF, et al. Lactobacillus acidophilus autolysins inhibit Helicobacter pylori in vitro[J]. Curr Micro-biol, 2001, 42(1):39-44. |
| [15] |
Nedenskov P. Nutritional requirements for growth of Helicobacter pylori[J]. Appl Environ Microbiol, 1994, 60(9):3450-3453.
doi: 10.1128/aem.60.9.3450-3453.1994 |
| [16] | Hayashi S, Sugiyama T, Asaka M, et al. Modification of Helicobacter pylori adhesion to human gastric epithelial cells by antiadhesion agents[J]. Dig Dis Sci, 1998, 43(Suppl 9):56S-60S. |
| [17] |
Dunne C, Dolan B, Clyne M. Factors that mediate colonization of the human stomach by Helicobacter pylori[J]. World J Gastroenterol, 2014, 20(19):5610-5624.
doi: 10.3748/wjg.v20.i19.5610 |
| [18] |
Slomiany BL, Slomiany A. Involvement of p38 MAPK-dependent activator protein (AP-1) activation in modulation of gastric mucosal inflammatory responses to Helicobacter pylori by ghrelin[J]. Inflammopharmacology, 2013, 21(1):67-78.
doi: 10.1007/s10787-012-0141-9 pmid: 22669511 |
| [1] | 闫辉,逄璐,李雪迎,杨文双,蒋世菊,刘平,闫存玲. 单中心就诊2~18岁儿童胆固醇水平异常发生率及病因分析[J]. 北京大学学报(医学版), 2022, 54(2): 217-221. |
| [2] | 张宏,董继元,王建军,范临夏,曲强,刘洋. 兰州市臭氧对儿童哮喘的短期影响及其季节性差异[J]. 北京大学学报(医学版), 2022, 54(2): 227-235. |
| [3] | 冯莎蔚,国慧,王勇,赵一姣,刘鹤. 乳牙数字化参考牙冠模型的初步构建[J]. 北京大学学报(医学版), 2022, 54(2): 327-334. |
| [4] | 刘雅菲,宋琳琳,邢茂炜,蔡立新,王东信. 全身麻醉下小儿开颅术术中心脏前负荷动态指标的一致性分析[J]. 北京大学学报(医学版), 2021, 53(5): 946-951. |
| [5] | 陈曼曼,杨招庚,苏彬彬,李艳辉,高迪,马莹,马涛,董彦会,马军. 中山市儿童青少年青春期身高生长突增规律[J]. 北京大学学报(医学版), 2021, 53(3): 506-510. |
| [6] | 杨雪,孙伟,王哲,姬爱平,白洁. 儿童和青少年牙外伤急诊患者临床分析[J]. 北京大学学报(医学版), 2021, 53(2): 384-389. |
| [7] | 张子一,夏斌,徐明明,李毅萍,唐瞻贵,陈泳清. 湖南韶山地区儿童口腔卫生干预效果评价[J]. 北京大学学报(医学版), 2020, 52(5): 913-918. |
| [8] | 陈小贤,钟洁,闫文娟,张红梅,姜霞,黄芊,薛世华,刘星纲. 树脂冠修复乳前牙的临床效果评价[J]. 北京大学学报(医学版), 2020, 52(5): 907-912. |
| [9] | 李军,顾芳,李在玲,吕愈敏. 北京单中心回顾性分析儿童肠镜临床特点及疾病谱12年演变[J]. 北京大学学报(医学版), 2019, 51(5): 819-823. |
| [10] | 陶春燕,李红霞,李雪迎,唐朝枢,金红芳,杜军保. 体位性心动过速综合征儿童及青少年在直立试验中血流动力学变化[J]. 北京大学学报(医学版), 2019, 51(3): 414-421. |
| [11] | 高玲,刘云,杨旭东. 鼻喷右美托咪定在儿童口腔门诊全身麻醉前的镇静效果[J]. 北京大学学报(医学版), 2018, 50(6): 1078-1082. |
| [12] | 吴鹏辉,谢瑶,赵卫红,华瑛,孙青,李硕,吴晔,卢新天. 血液病/肿瘤患儿并发可逆性后部白质脑病综合征[J]. 北京大学学报(医学版), 2018, 50(4): 662-665. |
| [13] | 董彦会,宋逸,董彬,邹志勇,王政和,杨招庚,王西婕,李艳辉,马军. 2014年中国7~18岁学生血压状况与营养状况的关联分析——基于中国儿童青少年血压评价标准[J]. 北京大学学报(医学版), 2018, 50(3): 422-428. |
| [14] | 陈天麒,董彬,张文静,高迪思,董彦会,马军,马迎华. 儿童青少年睡眠时间与速度和耐力成绩的相关性研究[J]. 北京大学学报(医学版), 2018, 50(3): 429-435. |
| [15] | 王潇,王欣,秦满. 唾液基质金属蛋白酶2、9与儿童龋病相关性的初步研究[J]. 北京大学学报(医学版), 2018, 50(3): 527-531. |
|