北京大学学报(医学版) ›› 2023, Vol. 55 ›› Issue (2): 357-361. doi: 10.19723/j.issn.1671-167X.2023.02.023
实性肺胎盘样变形1例及文献复习
- 1. 北京市密云区医院病理科, 北京 101500
2. 北京市密云区医院胸外科, 北京 101500
3. 北京大学第一医院病理科, 北京 100034
Solid placental transmogrification of the lung: A case report and literature review
Xue-mei HA1,Yong-zheng YAO2,Li-hua SUN1,Chun-yang XIN1,Yan XIONG3,*( )
)
- 1. Department of Pathology, Miyun District Hospital of Beijing, Beijing 101500, China
2. Department of Thoracic Surgery, Miyun District Hospital of Beijing, Beijing 101500, China
3. Department of Pathology, Peking University First Hospital, Beijing 100034, China
摘要:
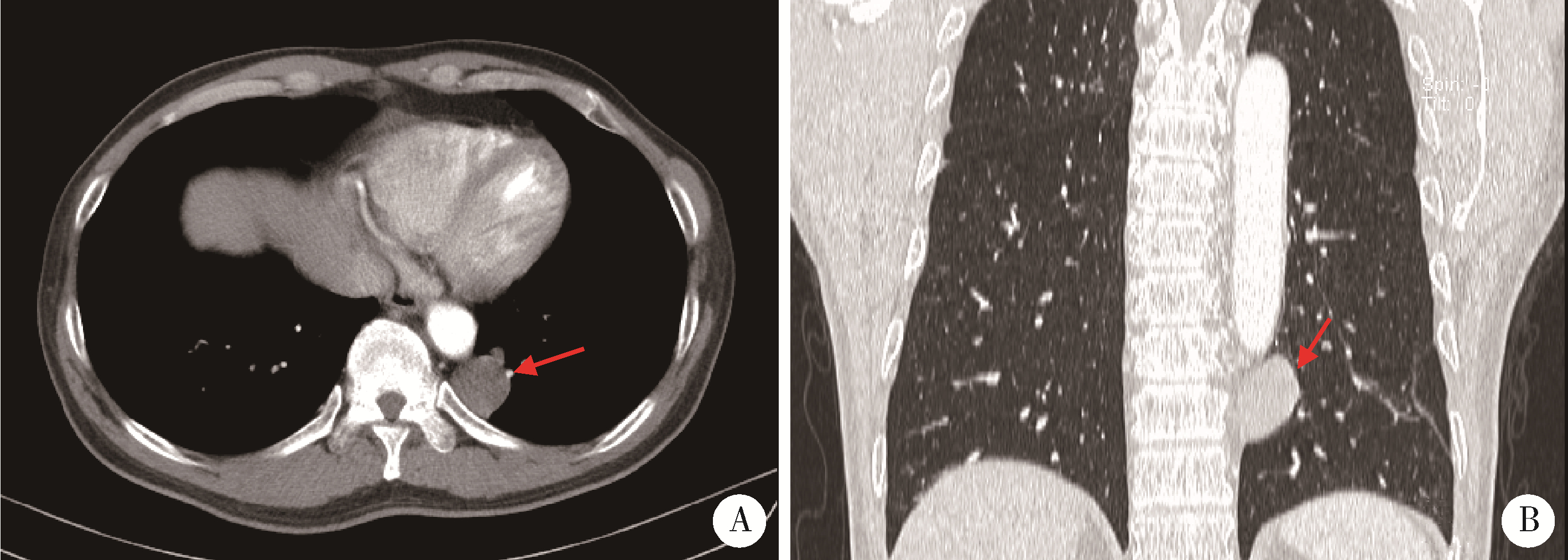
肺胎盘样变形(placental transmogrification of the lung, PTL)是一种非常罕见的良性肺部病变, 国内外文献报道仅40多例, 影像和组织形态学表现多样, 多为单侧囊性病变, 少数为实性结节。由于实性PTL极为罕见, 大多数病理医生和外科医生对其认识不足。本研究报道1例位于前纵隔以实性结节为特点的PTL, 并结合文献复习, 以提高广大医生对这一罕见病的认识。患者为男性, 52岁, 无吸烟史, 无明显临床症状。体检胸部CT发现左肺下叶后基底段脊柱旁有一个圆形低密度灶, 最大径约2.9 cm, 于胸腔镜下行肺段楔形切除送检。大体形态: 肺膜下肺实质内类圆形结节, 大小3.0 cm×3.0 cm×1.6 cm, 切面灰白, 海绵状, 质软。镜下可见结节由乳头状结构组成, 低倍镜下形似胎盘绒毛。乳头轴心间质水肿, 粉染无定形物及钙盐沉积, 少量淋巴细胞浸润, 毛细血管充血明显; 其间可见形态温和的圆形细胞增生、聚集, 细胞质浅染或空泡状, 细胞核圆形或卵圆形, 与脂肪细胞移行过渡, 免疫组织化学染色CD10阳性; 乳头表面被覆单层立方上皮, 无异型性, 无核分裂象。术后患者随访1年, 无复发及不适。该病的发病机制目前尚不清楚, 有错构瘤、肺气肿变异和间质细胞克隆性增生等多种假说。结合本病例并在文献复习的基础上, 我们推测PTL的发病机制可能为, 肺泡间隔内不成熟间质细胞增生并向各类间叶细胞分化, 随着病变的不断发展, 因阻塞活瓣效应或增生组织的牵拉作用引起继发性囊性变, 最终出现典型的单侧囊性结节。外科手术是PTL确诊和治疗的唯一选择, 临床医生可依据临床表现、PTL发生的部位及范围制定个体化手术方案, 在完整切除病灶的同时尽量保留周围正常肺组织, 术后预后良好。
中图分类号:
- R734.2
| 1 | McChesney T . Placental transmogrification of the lung: A unique case with remarkable histopathologic features[J]. Lab Invest, 1979, 40, 245- 246. |
| 2 |
Mark EJ , Muller KM , McChesney T , et al. Placentoid bullous lesion of the lung[J]. Hum Pathol, 1995, 26 (1): 74- 79.
doi: 10.1016/0046-8177(95)90117-5 |
| 3 |
Saito Y , Ikeya T , Hoshi E , et al. Placental transmogrification of the lung presenting as a small solitary nodule[J]. Ann Thorac Surg, 2009, 87 (3): 950- 952.
doi: 10.1016/j.athoracsur.2008.07.063 |
| 4 |
Ma DJ , Liu HS , Li SQ , et al. Placental transmogrification of the lung: Case report and systematic review of the literature[J]. Medicine (Baltimore), 2017, 96 (35): e7733.
doi: 10.1097/MD.0000000000007733 |
| 5 |
Yang M , Zhang XT , Liu XF , et al. Placental transmogrification of the lung presenting as a peripheral solitary nodule in a male with the history of trauma: A case report[J]. Medicine (Baltimore), 2018, 97 (18): e0661.
doi: 10.1097/MD.0000000000010661 |
| 6 |
Foschini G , Rodríguez CM , Rubio MM , et al. Placental transmogrification of the lung[J]. Arch Bronconeumol, 2022, 58 (5): 433.
doi: 10.1016/j.arbres.2021.04.004 |
| 7 |
Kim JW , Park IH , Kwon W , et al. Placental transmogrification of the lung[J]. Korean J Radiol, 2013, 14 (6): 977- 980.
doi: 10.3348/kjr.2013.14.6.977 |
| 8 |
Cavazza A , Lantuejoul S , Sartori G , et al. Placental transmogrification of the lung: Clinicopathologic, immunohistochemical and molecular study of two cases, with particular emphasis on the interstitial clear cells[J]. Hum Pathol, 2004, 35 (4): 517- 521.
doi: 10.1016/j.humpath.2003.10.023 |
| 9 |
Ferretti GR , Kocier M , Moro-Sibilot D , et al. Placental transmogrication of the lung: CT-pathologic correlation of a rare pulmonary nodule[J]. AJR Am J Roentgenol, 2004, 183 (1): 99- 101.
doi: 10.2214/ajr.183.1.1830099 |
| 10 |
Xu R , Murray M , Jagirdar J , et al. Placental transmogrification of the lung is a histologic pattern frequently associated with pulmonary fibrochondromatous hamartoma[J]. Arch Pathol Lab Med, 2002, 126 (5): 562- 566.
doi: 10.5858/2002-126-0562-PTOTLI |
| 11 |
Shapiro M , Vidal C , Lipskar AM , et al. Placental transmogrification of the lung presenting as emphysema and a lung mass[J]. Ann Thorac Surg, 2009, 87 (2): 615- 616.
doi: 10.1016/j.athoracsur.2008.05.076 |
| 12 | Narula N , Ngu S , Sharma D , et al. Placental transmogrification of the lung associated with unilateral pleural effusion: A case report with a comprehensive review of the literature[J]. Respir Med Case Rep, 2019, 26, 161- 164. |
| 13 |
Marchevsky AM , Guintu R , Koss M , et al. Swyer-James (MacLeod) syndrome with placental transmogrification of the lung: A case report and review of the literature[J]. Arch Pathol Lab Med, 2005, 129 (5): 686- 689.
doi: 10.5858/2005-129-0686-SMSWPT |
| 14 |
Hamza A , Khawar S , Khurram MS , et al. Pulmonary placental trans-mogrification associated with adenocarcinoma of the lung: A case report with a comprehensive review of the literature[J]. Autops Case Rep, 2017, 7 (3): 44- 49.
doi: 10.4322/acr.2017.027 |
| 15 | Mudawi D , Touqeer R , Montero-Fernandez A , et al. Image of the month: Placental transmogrification of the lung: A rare cause of cystic lung disease[J]. Clin Med (Lond), 2020, 20 (6): 603- 604. |
| 16 |
Jenkins JM , Attia RQ , Green A , et al. A case of pulmonary placental transmogrification[J]. Asian Cardiovasc Thorac Ann, 2016, 24 (8): 811- 813.
doi: 10.1177/0218492316661462 |
| 17 |
Horsley WS , Gal AA , Mansour KA , et al. Unilateral giant bullous emphysema with placental transmogrification of the lung[J]. Ann Thorac Surg, 1997, 64 (1): 226- 228.
doi: 10.1016/S0003-4975(97)00274-9 |
| 18 |
Fidler ME , Koomen M , Sebek B , et al. Placental transmogrification of the lung, a histologic variant of giant bullous emphysema. Clinicopathological study of three further cases[J]. Am J Surg Pathol, 1995, 19 (5): 563- 570.
doi: 10.1097/00000478-199505000-00008 |
| 19 |
Moneghini L , Tosi D , Graziani D , et al. CD10 and CD34 as markers in vascular malformations with PIK3CA and TEK mutations[J]. Hum Pathol, 2020, 99, 98- 106.
doi: 10.1016/j.humpath.2020.04.001 |
| 20 |
Kadota K , Buitrago D , Lee MC , et al. Tumoral CD10 expression correlates with high-grade histology and increases risk of recurrence in patients with stage Ⅰ lung adenocarcinoma[J]. Lung Cancer, 2015, 89 (3): 329- 336.
doi: 10.1016/j.lungcan.2015.06.003 |
| [1] | 钟华, 李原, 徐丽玲, 白明欣, 苏茵. 18F-FDG PET/CT在风湿免疫病中的应用[J]. 北京大学学报(医学版), 2024, 56(5): 853-859. |
| [2] | 周庆欣,杨晴晴,石舒原,李沛,孙凤. 健康体检人群血尿酸与气流阻塞的相关性[J]. 北京大学学报(医学版), 2024, 56(4): 693-699. |
| [3] | 李文根,古晓东,翁锐强,刘苏东,陈超. 血浆外泌体miR-34-5p和miR-142-3p在系统性硬化症中的表达及临床意义[J]. 北京大学学报(医学版), 2023, 55(6): 1022-1027. |
| [4] | 王子恺,莫佳丽,张蒙,廖纪萍. 2013—2020年北京市慢性阻塞性肺疾病急性加重女性住院患者的流行病学和住院费用分析[J]. 北京大学学报(医学版), 2023, 55(6): 1074-1081. |
| [5] | 沈棋,刘亿骁,何群. 肾黏液样小管状和梭形细胞癌的临床病理特点及预后[J]. 北京大学学报(医学版), 2023, 55(2): 276-282. |
| [6] | 侯卫华,宋书杰,石中月,金木兰. 幽门螺杆菌阴性早期胃癌的临床病理特征[J]. 北京大学学报(医学版), 2023, 55(2): 292-298. |
| [7] | 农琳,王微,梁丽,李东,李鑫,李挺. 母细胞性浆样树突细胞肿瘤13例临床病理学特征[J]. 北京大学学报(医学版), 2023, 55(2): 308-314. |
| [8] | 宁博涵,张青霞,杨慧,董颖. 伴间质细胞增生、玻璃样变性及索状结构的子宫内膜样腺癌1例[J]. 北京大学学报(医学版), 2023, 55(2): 366-369. |
| [9] | 博尔术,洪鹏,张宇,邓绍晖,葛力源,陆敏,李楠,马潞林,张树栋. 乳头状肾细胞癌的临床病理特征和预后分析[J]. 北京大学学报(医学版), 2022, 54(4): 615-620. |
| [10] | 吴燕芳,高飞,林滇恬,陈志涵,林禾. 托法替布联合治疗抗MDA5抗体阳性的无肌病皮肌炎并发快速进展型间质性肺病5例临床分析[J]. 北京大学学报(医学版), 2021, 53(5): 1012-1016. |
| [11] | 夏芳芳,鲁芙爱,吕慧敏,杨国安,刘媛. 系统性红斑狼疮伴间质性肺炎的临床特点及相关因素分析[J]. 北京大学学报(医学版), 2021, 53(2): 266-272. |
| [12] | 包海荣,刘晓菊,谭恩丽,舒娟,董继元,李盛. 兰州市气温和相对湿度对慢性阻塞性肺疾病患者门诊就诊人次的影响及其交互效应[J]. 北京大学学报(医学版), 2020, 52(2): 308-316. |
| [13] | 谢江,李菲. 睡眠重叠综合征与糖尿病发病率的横断面调查研究[J]. 北京大学学报(医学版), 2019, 51(2): 252-255. |
| [14] | 卢昕,张立宁. 肌活检在特发性炎性肌病诊断和临床分型中的价值[J]. 北京大学学报(医学版), 2018, 50(6): 949-951. |
| [15] | 黄子雄,杜依青,张晓鹏,刘士军,徐涛. 肾细胞癌骨转移的临床与病理分析[J]. 北京大学学报(医学版), 2018, 50(5): 811-815. |
|
||