目的 探讨尿路上皮癌相关1(urothelial carcinoma-associated 1, UCA1)基因及 miR-18a在雌激素受体(estrogen receptor, ER)阳性的乳腺癌细胞他莫昔芬耐药中的作用和调控机制。方法 通过反转录实时定量PCR测定乳腺癌细胞中 UCA1和 miR-18a的表达,用双荧光素酶报告实验验证 miR-18a和 UCA1 3'UTR区域结合。在他莫昔芬敏感的MCF-7细胞中过表达 UCA1或敲减 miR-18a,在他莫昔芬耐药的LCC9和BT474细胞中敲减 UCA1或过表达 miR-18a,CCK-8方法测定细胞活力,软琼脂克隆形成实验测定细胞克隆形成能力,流式细胞术测定细胞周期。结果 他莫昔芬耐药的LCC2、LCC9和BT474细胞中, UCA1表达量显著高于他莫昔芬敏感的MCF-7细胞。MCF-7细胞在0.1 μmol/L 他莫昔芬处理后, UCA1的表达水平显著升高。 UCA1过表达提高了MCF-7细胞在他莫昔芬处理后的活力,而 UCA1 siRNA则显著降低了LCC9和BT474细胞在他莫昔芬处理后的活力。在MCF-7细胞中,相对于质粒对照+他莫昔芬组, UCA1+他莫昔芬组的克隆数目多19.0%,克隆平均大小增加29.0%,G1期细胞比例减少7.3%,S期细胞增加6.7%。在BT474细胞中,相对于siRNA对照+他莫昔芬组, si-UCA1+他莫昔芬组的克隆数目少54.0%,克隆平均大小减少42.0%,G1期细胞比例增加9.0%,S期细胞减少6.2%。 UCA1有一个 miR-18a结合位点,敲减 miR-18a提高了MCF-7细胞在他莫昔芬处理后的活力,过表达 miR-18a则显著降低了BT474细胞在他莫昔芬处理后的活力。在MCF-7细胞中,相对于对照+他莫昔芬组, miR-18a抑制+他莫昔芬组的克隆数目多15.0%,克隆平均大小增加33.0%,G1期细胞比例减少8.8%,S期细胞增加5.3%。在BT474细胞中,相对于对照+他莫昔芬组, miR-18a过表达+他莫昔芬组的克隆数目少47.0%,克隆平均大小减少25.0%,G1期细胞比例增加13.3%,S期细胞减少7.9%。结论 UCA1可以通过竞争性抑制 miR-18a增强乳腺癌细胞的他莫昔芬治疗耐药。
Objective: To investigate how urothelial carcinoma-associated 1 ( UCA1) and miR-18a modulates acquired tamoxifen resistance and the relevant mechanisms in estrogen receptor (ER) positive cancer cells.Methods: qRT-PCR was performed to detect UCA1 and miR-18a expression in breast cancer cells. Dual luciferase assay was performed to detect the binding between miR-18a and UCA1 3'UTR. Tamoxifen sensitive MCF-7 cells were transfected with UCA1 expression vector or miR-18a inhi-bitors. Tamoxifen resistant LCC9 and BT474 cells were transfected with UCA1 siRNA or miR-18a mi-mics. CCK-8 assay was performed to detect cell viability. Soft agar assay was performed to assess cell colony formation. Flow cytometric analysis was performed to check cell cycle distribution.Results: UCA1 was significantly upregulated in tamoxifen resistant LCC2, LCC9, and BT474 cells than in tamoxifen sensitive MCF-7 cells. UCA1 expression was significantly upregulated in MCF-7 cells after treatment with 0.1 μmol/L tamoxifen. UCA1 overexpression enhanced cell viability of MCF-7 cells after tamoxifen treatment, while UCA1 siRNA significantly suppressed viability of LCC9 and BT474 cells after tamoxifen treatment. In MCF-7 cells, compared with vector control+tamoxifen group, the average cell colony number and colony size of the UCA1+tamoxifen group was 19.0% more and 29.0% larger respectively, while the proportions of the cells in G1 phase and in S phase were 7.3% lower and 6.7% higher respectively. In BT474 cells, compared with siRNA control+tamoxifen group, the average cell colony number and colony size of the si-UCA1+tamoxifen group were 54.0% less and 42.0% smaller respectively, while the proportions of the cells in G1 phase and in S phase were 9.0% higher and 6.2% lower respectively. UCA1 directly interacted with miR-18a and reduced its expression in ER positive breast cancer cells. Knockdown of miR-18a increased viability of MCF-7 cells after tamoxifen treatment, while miR-18a overexpression significantly reduced viability of BT474 cells after tamoxifen treatment. In MCF-7 cells, compared with miRNA inhibitor control+tamoxifen group, the average cell colony number and colony size of the miR-18a inhibitor+tamoxifen group were 15.0% more and 33.0% larger respectively, while the proportions of the cells in G1 phase and in S phase were 8.8% lower and 5.3% higher respectively. In BT474 cells, compared with miRNA control+tamoxifen group, the average cell colony number and colony size of the miR-18a mimics+tamoxifen group were 47.0% less and 25.0% smaller respectively, while the proportions of the cells in G1 phase and in S phase were 13.3% higher and 7.9% lower respectively.Conclusion: UCA1 can increase tamoxifen resistance of ER positive breast cancer cells via competitively inhibiting of miR-18a.
乳腺癌是女性最常见的恶性肿瘤之一, 也是女性肿瘤患者中死亡率第二位的肿瘤[1]。在所有乳腺癌肿瘤中, 大约70%为雌激素受体(estrogen receptor, ER)阳性[2, 3], 因此, 他莫昔芬作为雌二醇竞争性拮抗剂, 被广泛用于ER阳性的早期乳腺癌患者术后的辅助治疗[4]。虽然他莫昔芬的临床使用显著提高了患者的生存期, 但是乳腺癌细胞通常会在一段时间治疗后出现他莫昔芬耐药, 从而导致肿瘤复发和他莫昔芬治疗失败[4]。他莫昔芬的耐药机制十分复杂, 很多分子机制目前仍然未知。
长链非编码RNA(long noncoding RNA, lnc-RNA)是一类转录本长度超过200个核苷酸的RNA分子, 并不具有编码蛋白的能力[5]。最近的研究发现, lncRNA可以参与许多信号通路的调控, 从而促进或者抑制肿瘤[6, 7, 8], 也有研究报道, lncRNA在乳腺癌的内分泌治疗中可能起到比较关键的调控作用[9, 10], 比如在他莫昔芬敏感的ZR-75-1细胞中过表达乳腺癌抗雌激素药物耐药性基因4(breast cancer anti-estrogen resistance 4, BCAR4)可以部分解除他莫昔芬对细胞增殖的抑制作用[10], 因此, BCAR4也被认为是乳腺癌他莫昔芬耐药的标志物之一[9, 11]。另外一项研究发现, 长链基因间非编码RNA1016和00160(LINC01016和LINC00160)是直接的ERα 转录调控靶点。LINC01016和LINC00160的表达与ERα 的表达呈正相关, 且LINC01016和LINC00160的高表达预示了患者的不良预后[12]。在乳腺细胞中敲减LINC00160可以显著降低细胞增殖水平[12]。lncRNA HOTAIR上调可以促进非配体依赖的ER活性并且促进他莫昔芬耐药[13]。
尿路上皮癌相关1(urothelial carcinoma-associa-ted 1, UCA1)基因是在膀胱移行上皮细胞癌中发现并命名的lncRNA[14]。UCA1有3个外显子, 编码两个转录本, 包括一个1.4 kb和一个2.2 kb的转录本, 1.4 kb的转录本包括在2.2 kb的转录本中[15]。最近的研究证实, 1.4 kb的UCA1转录本在乳腺癌中是一个原癌基因, UCA1在ER阳性及ER阴性的乳腺癌细胞中的表达均显著高于正常乳腺细胞[15, 16, 17]。功能上, UCA1可以通过与核不均一核糖核蛋白Ⅰ (heterogeneous nuclear ribonucleo protein, hnRNP Ⅰ )形成复合物来降低p27的表达[15]。UCA1也可通过分子海绵效应降低miR-143的表达来调控ER阳性乳腺癌细胞的生长和凋亡[18]。除此以外, UCA1也可以调控ER阳性MCF-7细胞的阿霉素敏感性[19], UCA1上调可以促进ER阴性的MDA-MB-231细胞上皮间质转化[16]。但是, 目前有关UCA1表达与ER阳性乳腺癌细胞的他莫昔芬敏感性关系的报道较少, 本研究旨在明确UCA1对ER阳性乳腺癌细胞的他莫昔芬敏感性调控, 进而探究下游的分子机制, 为乳腺癌精准治疗提供实验依据。
ER阳性且他莫昔芬敏感的乳腺癌细胞MCF-7, ER阳性、人类表皮生长因子受体2(human epidermal growth factor receptor-2, HER2)阳性且他莫昔芬耐药的乳腺癌细胞BT-474均购自美国ATCC细胞库。基于MCF-7诱导的他莫昔芬耐药的LCC2和LCC9细胞均购自中国科学院上海生命科学研究院细胞资源中心。肿瘤细胞用RMPI-1640培养基培养, 培养基中添加10%(体积分数)胎牛血清(fetal bovine serum, FBS)、2 mmol/L谷氨酰胺、100 U/mL青霉素和100 mg/L链霉素。
以MCF-7的总RNA为模板, 使用反转录试剂盒(Promega)进行反转录PCR获得1.4 kb的UCA1序列。UCA1的引物序列为:正向引物5'-CGCGGATCCTTTATCAGGCATATTAGCTTTAA-3', 反向引物5'-GCGAATTCTGACATTCTTCTGGACAATG-3'。把序列克隆到pcDNA3.1(+) (Invitrogen) 的EcoR Ⅰ 和BamR Ⅰ 位点之间, 重组质粒命名为pc-DNA3.1-UCA1。将重组质粒转染到MCF-7细胞中实现UCA1过表达, 转染试剂为Lipofectamie 2000 (Invitrogen)。
miR-18a模拟物 (mimics)、miR-18a抑制物 (inhibitors, IH)、UCA1小干扰RNA(small interfe-ring RNA, siRNA)和对应的阴性对照全部购自广州市锐博生物科技有限公司。在准备miR-mimics和siRNA转染实验的12 h前, 将所需细胞按30%密度接种于培养皿中。参考转染试剂Lipofectamie 2000 (Invitrogen) 说明书将miR-mimics或siRNA和转染试剂分别加入无血清DMEM培养基中, 静置5 min后混合, 再次静置20 min, 将混合好的DMEM培养基加入换为新培养基含血清的细胞培养皿中, 转染24 h后更换新培养基, 之后按需要浓度加入他莫昔芬, 处理指定时间后收集细胞。
联合使用PITA (http://genie.weizmann.ac.il/pubs/mir07/mir07_prediction.html)和RNAhybrid (http://bibiserv2.cebitec.uni-bielefeld.de/rnahybrid)来预测UCA1可能的miRNA结合位点。
按照TRIzolRNA提取试剂盒说明书提取细胞总RNA, 以细胞总RNA为模板, 应用PrimeScript® 逆转录试剂盒(TaKaRa)或TaqMan miRNA逆转录试剂盒(Applied Biosystems)将RNA逆转录为cDNA。根据qRT-PCR试剂盒说明书和基因对应的引物对cDNA进行扩增。UCA1的引物为:正向引物5'-CTCTCCATTGGGTTCACCATTC-3', 反向引物5'-GCGGCAGGTCTTAAGAGATGAG-3'。用TaqMan miRNA分析试剂盒检测miR-18a的表达, 使用U6作为内参基因。所有qRT-PCR实验均使用Applied Biosystems 7500 实时定量PCR仪进行, RNA的相对表达量使用2-Δ Δ CT进行计算。
取转染后24 h的MCF-7细胞, 调整细胞密度为3× 104个/mL, 取96孔板每孔接种100 μ L细胞悬液, 放入培养箱继续培养24 h, 之后将培养基换为含不同浓度他莫昔芬(0.1、0.5、1.0、5.0、10.0、15.0、20.0、50.0 μ mol/L)的培养基。细胞继续培养5 d后进行CCK-8检测, 检测时每孔加10 μ L CCK-8检测试剂, 放于37 ℃、5%(体积分数)CO2培养箱中孵育2 h。用酶标仪测定450 nm波长的光密度值(
将1.2%(质量分数)低熔点琼脂糖与2× 细胞培养基以体积比1 :1混合制备0.6%(质量分数)的底层琼脂, 6孔板中每个孔加0.8 mL, 4℃凝固。取对数期细胞, 胰酶消化后吹散成单个细胞悬液, 计数。将0.6%低熔点琼脂糖与2× 细胞培养基以体积比1 :1混合, 制备0.3%(质量分数)的上层琼脂, 每孔加1 mL上层琼脂和单细胞悬液(约5× 104细胞/孔), 混匀, 4℃ 5 min凝固。置于37 ℃、5% CO2培养箱中培养2周。细胞克隆用0.5%(体积分数)结晶紫染色, 计数含50个细胞以上的克隆, 计算细胞集落形成率=(克隆形成数/初始细胞数)× 100%。实验组及阴性对照组分别设3个复孔, 实验重复3次。
消化好的单细胞悬液先采用70%(体积分数)乙醇4℃固定过夜, 后用1× PBS洗涤3次。固定好的细胞用100 mg/L RNase A 37 ℃孵育30 min, 之后用10 mg/L propidium iodide (PI)混匀后4℃避光染色30 min。用流式细胞仪(FACSCaliber, USA) 488 nm激发波长检测DNA染色。实验组及阴性对照组分别设3个重复, 实验重复3次。
将含预测的野生型miR-18a结合位点的UCA1序列片段和含突变型miR-18a结合位点的UCA1片段分别插入荧光素酶报告质粒pmirGLO (Promega)的XhoⅠ 和XbaⅠ 位点之间, 重组质粒分别命名为pmirGLO-UCA1-WT和pmirGLO-UCA1-MT, 以表达海肾荧光素酶的载体pRL-TK作为内参对照, 将miR-18a mimics、mimics对照分别与荧光素酶报告载体共转染入MCF-7细胞中, 转染24 h后采用Promega公司的Dual-Lucifersae Reporter Assay System进行样品Lucifersae活性检测。
所有数据采用SPSS 18.0软件进行分析, 以均数± 标准差表示, 对多组数据采用单因素方差分析和Student-Newman-Keuls (SNK)法两两比较分析, 两组间数据比较采用t检验, P< 0.05为差异有统计学意义。
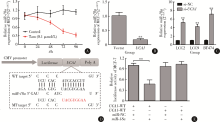
实时定量PCR结果显示, 他莫昔芬耐药的LCC2、LCC9和BT474细胞中UCA1表达量显著高于他莫昔芬敏感的MCF-7细胞(图1A)。MCF-7细胞在0.1 μ mol/L他莫昔芬处理后, UCA1的表达水平也显著升高, 第72小时升高了(7.7± 0.9)倍, 第96小时升高了(8.6± 1.1)倍(图1B)。
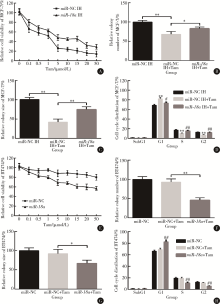
为了研究UCA1的他莫昔芬敏感性调节作用, 将MCF-7细胞转染UCA1过表达载体pcDNA3.1-UCA1, LCC2和BT474细胞转染UCA1 siRNA。细胞活力实验结果显示, UCA1过表达显著提高了MCF-7细胞在不同浓度他莫昔芬处理后的细胞活力(图2A), 而UCA1 siRNA则显著降低了LCC9和BT474细胞在他莫昔芬处理后的活力(图2B、C)。细胞克隆形成实验结果显示, 在MCF-7细胞中过表达UCA1, 可以部分解除他莫昔芬抑制克隆形成作用。相对于质粒对照+他莫昔芬组, UCA1+他莫昔芬组的克隆数目多19.0%, 克隆平均大小增加29.0%(图2D、E)。相反, 在BT474细胞中抑制UCA1可以显著增加他莫昔芬抑制克隆形成作用, 相对于siRNA对照+他莫昔芬组, si-UCA1+他莫昔芬组的克隆数目少54.0%, 克隆平均大小减少42.0% (图2D、E)。通过流式细胞术分析细胞周期结果表明, 在MCF-7细胞中过表达UCA1可以部分解除他莫昔芬诱导的G0/G1细胞周期阻滞。相对于质粒对照+他莫昔芬组, UCA1+他莫昔芬组G1期细胞比例减少7.3%, S期细胞增加6.7% (图2F、G)。相反, 在BT474细胞中抑制UCA1可以显著增加他莫昔芬诱导的G0/G1细胞周期阻滞, 相对于 siRNA对照+他莫昔芬组, si-UCA1+他莫昔芬组G1期细胞比例增加9.0%, S期细胞减少6.2% (图2F、G、H)。
MCF-7细胞在他莫昔芬处理后, miR-18a的表达水平显著下降(图3A), 在MCF-7细胞中过表达UCA1可以显著降低细胞中miR-18a的表达水平(图3B), 而在LCC2、LCC9和BT474细胞中, 敲减UCA1导致了4倍或以上的miR-18a表达增加(图3C)。生物信息学分析结果显示, UCA1可能有一个高度保守的miR-18a结合位点(图3D)。将野生型和突变型的UCA1片段插入pmirGLO荧光素酶表达载体后进行miR-18a结合实验结果显示, miR-18a可以显著抑制pmirGLO/UCA1-WT的荧光素酶表达, 但是并不能抑制pmirGLO/UCA1-MT的荧光素酶表达(图3E), 说明miR-18a可以结合预测的UCA1位点。
细胞活力和细胞克隆形成实验结果显示, 在MCF-7细胞中敲减miR-18a可以显著提高在不同浓度他莫昔芬处理后的细胞活力(图4A), 并且降低他莫昔芬对MCF-7细胞克隆数目及大小的抑制作用。相对于对照+他莫昔芬组, miR-18a抑制+他莫昔芬组的克隆数目多15.0%, 克隆平均大小增加33.0%(图4B、C)。敲减miR-18a也可以部分解除他莫昔芬诱导的G0/G1细胞周期阻滞。相对于对照+他莫昔芬组, miR-18a抑制+他莫昔芬组G1期细胞比例减少8.8%, S期细胞增加5.3%(图4D)。
在BT474细胞中转染miR-18a可以显著降低在不同浓度他莫昔芬处理后的细胞活力(图4E), 并且增加他莫昔芬对MCF-7细胞克隆数目及大小的抑制作用。相对于对照+他莫昔芬组, miR-18a过表达+他莫昔芬组的克隆数目少47.0%, 克隆平均大小减少25.0%(图4F、G)。过表达miR-18a可以提高他莫昔芬诱导的G0/G1细胞周期阻滞。相对于对照+他莫昔芬组, miR-18a过表达+他莫昔芬组G1期细胞比例增加13.3%, S期细胞减少7.9% (图4H)。
虽然ER阳性乳腺癌的内分泌治疗已经取得了很多进展, 但肿瘤对治疗产生的新的和获得性的耐药仍是重要的临床问题[20]。lncRNA对内分泌治疗的调控作用逐渐被人们所认知, 如BCAR4和HOTAIR的高表达均参与了乳腺癌细胞他莫昔芬耐药性的调控[10, 13]。以往的报道指出UCA1在乳腺癌中有明确的促癌作用[15, 17, 18], 并且UCA1也可以在多种肿瘤细胞中调控化疗敏感性, 如非小细胞肺癌细胞、乳腺癌细胞和肝癌细胞[19, 21, 22]。lncRNA的调控机制相当复杂, 且与下游分子参与的调控通路密切相关。
本研究发现, 他莫昔芬耐药的LCC2、LCC9和BT474细胞中的UCA1表达水平显著高于他莫昔芬敏感的MCF-7细胞。最近的另外一项研究也证实, 他莫昔芬耐药的乳腺癌细胞和其外泌体中的UCA1的表达水平上调[23]。功能上, UCA1过表达可以显著降低他莫昔芬敏感性, 而敲减UCA1则可以明显增加他莫昔芬敏感性。有研究指出, UCA1可以通过抑制mTOR信号途径增加他莫昔芬耐药性[24], 同时, 竞争性内源RNA也是lncRNA发挥其调控作用的重要机制。通过扮演分子海绵, lncRNA可以降低细胞内与其结合的miRNA水平, 并恢复miRNA抑制的信号通路[25]。在多种肿瘤细胞中, UCA1通过miRNA分子海绵作用调控了多种促癌通路, 如UCA1可以通过miR-145/ZEB1/2/FSCN1通路促进膀胱癌细胞迁移与侵袭[26], 也可以通过降低miR-193a-3p来促进非小细胞肺癌的发展[27], 还可以通过抑制miR-216b而激活FGFR1/ERK信号通路, 促进肝癌进展[28]。本研究发现, UCA1可以通过分子海绵作用降低miR-18a表达水平, 从而促进ER阳性乳腺癌细胞的他莫昔芬耐药性。
以前的研究报道, miR-18a在乳腺癌中起到肿瘤抑制分子作用。通过抑制HIF1-α 表达, miR-18a可以减少乳腺癌细胞肺转移[29], 更重要的是, miR-18a可以调控细胞周期进展。有报道指出, miR-18a可以抑制cdc42表达, 从而抑制肿瘤细胞的细胞周期进展, 减慢细胞增殖和侵袭[30], 因此, miR-18a很有可能是一个重要的UCA1下游细胞周期调控分子, 但是, 本文并未通过体外肿瘤模型对UCA1和miR-18a的他莫昔芬作用机制进行研究, 这是本研究的不足之处。UCA1在他莫昔芬处理后上调的机制还不清楚, 如是否受到ER信号途径调控也还未知, 除此以外, 由于lncRNA和miRNA均可以参与多信号途径调控, UCA1和miR-18a在乳腺癌中的调控网络仍需要更深入研究。
(本文编辑:任英慧)
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|