目的:通过研究跨膜蛋白CMTM2在睾丸和精子中的表达来探讨CMTM2蛋白在男性生殖系统中潜在的功能。方法:Western blot方法检测CMTM2在人睾丸及精子中的表达,免疫组织化学及组织免疫荧光方法检测CMTM2在人睾丸组织中的定位,TRITC-CMTM2及FITC-Hoechst免疫荧光双染法检测CMTM2在人精子顶体反应前后的定位。结果:CMTM2在人睾丸及精子中均有表达,在睾丸组织中CMTM2定位于各级生精细胞的细胞膜,睾丸组织冰冻切片免疫荧光检测的结果与免疫组织化学一致,进一步证实CMTM2存在于睾丸各级生精细胞的细胞膜,在精子中则定位于核后区附近。以TRITC-CMTM2及FITC-Hoechst免疫荧光双染法检测CMTM2在人精子顶体反应前后的定位,在顶体反应前后,CMTM2在人精子的定位及表达量未发生改变。结论:CMTM2在人睾丸和精子中均有表达,其表达定位具有细胞特异性和区域特异性,提示其可能在人睾丸精子发生和精卵融合过程中发挥作用,是男性不育研究的靶基因。CMTM2在男性生殖系统中的表达呈现细胞与生精区域的特异性,提示其高度参与生精功能,但目前针对CMTM2在男性生殖系统与精子生成过程中的作用仍未明确,今后可以采用体外受精-胚胎移植等技术进一步研究CMTM2的作用。
Objective:To study the expression of transmembrane protein CMTM2 in the testis and sperm of adult males and to approach the potential function of the protein in the male reproductive system.Methods:The expression of CMTM2 in human testis and sperm was confirmed by Western blot. Immunohistochemical staining was used for detecting CMTM2 localization in the testis tissue, TRITC-CMTM2 and FITC-Hoechst double immunofluorescence staining was performed to examine the subcellular localization of CMTM2 in the human sperm before and after acrosome reaction, that is, immunofluorescent staining was used for detecting CMTM2 localization in both the testis and sperm before and after the acrosome reaction.Results:CMTM2 was presented in both human testis and sperm. In the testis, CMTM2 immunoreactive particles were observed mainly in the membrane of the different stages of spermatogenic cells. In the human sperm, its immunoreactivity was restrictively localized to the posterior head where sperm-egg fusion occurred, and the CMTM2 localization was not affected by sperm acrosome reaction. CMTM2 was widely expressed in seminiferous tubules of the human testis, mainly in the cell membranes of spermatogenic cells, which was consistent with the previous reports. The immunofluorescence performed on frozen human testis slides showed similar findings with immunohistochemistry, which gave weight to the localization of CMTM2 in the cell membranes of spermatogenic cells at different stages. TRITC-CMTM2 and FITC-Hoechst double immunofluorescence staining was performed to examine the subcellular localization of CMTM2 in the human sperm before and after acrosome reaction. CMTM2 was localized at the posterior head of sperm before and after acrosome reaction. The localization and expression of CMTM2 were not affected by sperm acrosome reaction.Conclusion:Expression of CMTM2 in the male reproductive system of the adult human exhibits cell- and region-specific patterns, which suggests that they may play an important role in spermatogenesis and sperm-egg fusion. The expression of CMTM2 in the male reproductive system of the adult human exhibits cell- and region-specific patterns, which suggests that they may play an important role in spermatogenesis and sperm-egg fusion. However, it still remains to be further elucidated about the definite role of CMTM2 in male reproductive system and the process of spermatogenesis. And in vitro fertilization experiments are needed to confirm the role of CMTM2 in fertilization in future.
睾丸中精子发生及受精是一个受到高度调控的过程, 不仅受到睾丸生精细胞自身因素的作用, 还有一系列生物因子的参与。CMTM2(CKLF-like MARVEL transmembrane domain-containing protein 2)是新近发现的CMTM家族成员, CMTM2蛋白存在跨膜和分泌两种形式, 其结构和功能介于趋化因子和四次跨膜蛋白之间[1](图1)。CMTM2蛋白在睾丸组织中呈现高表达, 既往的研究也发现CMTM2表达异常会造成生精过程障碍[2, 3], 这些都提示CMTM2可能在生精过程中发挥着重要的作用。目前只有少数文献报道证实了CMTM2蛋白在生殖系统中的作用且研究并不深入。本研究采用 Western blot、免疫组织化学及免疫荧光技术在蛋白水平上研究CMTM2在人睾丸及顶体反应前后的精子中的表达与定位情况, 旨在初步探讨其在睾丸精子发生和受精过程中潜在的重要作用, 为进一步明确其在男性生殖过程中的功能提供理论依据和实验资料。
材料试剂有兔多克隆CMTM2抗体(Abcam, Inc, USA), 辣根过氧化物酶、FITC、TRITC标记的山羊抗兔IgG和DAB显色试剂盒(北京中山金桥生物技术有限公司), FITC标记的PSA及A23187(Sigma, Inc, USA), 人输卵管培养液(human tubal fluid, HTF, In Vitro care, Inc, USA)。
人睾丸组织标本取自北京大学人民医院生殖医学中心行睾丸穿刺的10名梗阻性无精子症患者, 年龄20~45岁, 已婚且经随访至少育有一个后代。精子标本由北京大学人民医院生殖医学中心男科提供, 采集30名健康男子精液, 经常规分析精液的各项指标符合世界卫生组织(World Health Organization, WHO)《人类精液检验与处理实验室手册》(第五版)标准。本研究经过北京大学人民医院医学伦理委员会批准, 所取标本均获得本人知情同意。
将精液置于37 ℃水浴30 min使之液化, 取1 mL 60%(体积分数)的Percoll 置于无菌试管中, 置温箱中预热, 将已液化的精液混匀, 取1 mL小心置于预热好的60%的Percoll液面上, 350× g离心10 min, 收集沉淀的活精子, 用HTF洗涤2次。
为检测CMTM2在人睾丸及精子中的表达, 将人睾丸蛋白和精子蛋白用SDS-PAGE分离, 转膜后用体积比为1 ∶ 200的兔多抗4 ℃孵育过夜, 用体积比为1 ∶ 2 000的辣根过氧化物酶(horseradish pe-roxidase, HRP)标记的羊抗兔IgG, 37 ℃孵育1 h, 用增强化学发光法(enhanced chemiluminescence, ECL)检测试剂盒显色, Fluor Chem 5500(Alpha Innotech)成像系统检测杂交信号。
人睾丸组织用Bouin’ s液固定, 石蜡包埋, 切成5 μ m的薄片贴附于硅烷化的载玻片上, 切片经脱蜡、水化后用3%(体积分数)H2O2处理以去除内源性过氧化物酶。加入EDTA进行抗原修复, 0.01 mol/L PBS洗涤后, 用进口羊血清工作液室温封闭2 h后加入CMTM2抗体(体积比1 ∶ 100稀释于一抗稀释液), 置于湿盒中4 ℃过夜, 0.01 mol/L PBS洗涤后, 加入HRP标记的羊抗兔IgG(体积比1 ∶ 1 000), 室温孵育1 h, 再次洗涤后用DAB试剂显色, 封片后用Axioskop 2 plus(ZEISS, Germany)显微镜观察, 以细胞中有棕黄色颗粒为阳性反应。上述实验步骤中用兔IgG作为阴性对照。
人睾丸组织用OCT包埋(optimal cutting temperature compound), 用冰冻切片机切成5 μ m的冰冻切片贴于载玻片上。切片用4%(体积分数)多聚甲醛溶液室温固定30 min, 封闭液封闭2 h, 加入CMTM2兔多抗(体积比1 ∶ 100)4 ℃孵育过夜, PBS洗3次, 加入体积比为1 ∶ 100的FITC标记的羊抗兔IgG, 37 ℃孵育1 h, PBS洗后以10 mg/L Hoechst 33258染核30 s, 用含50%(体积分数)甘油的磷酸盐缓冲液封片观察。将精子悬液置于CO2培养箱中在37 ℃及5%(体积分数)CO2条件下培养5 h使精子获能。获能后的精子悬液中加入溶于DMSO的钙离子载体A23187, 终浓度为10 μ mol/L, 37 ℃及5%CO2继续培养30 min以诱导顶体反应。取顶体反应前后的精子20 μ L涂于载玻片上, 自然晾干后放置于4%多聚甲醛溶液室温固定30 min, 封闭液封闭2 h, 加入CMTM2兔多抗(体积比1 ∶ 100)4 ℃孵育过夜, PBS洗3次, 加入体积比为1 ∶ 100的TRITC标记的羊抗兔IgG, 37 ℃孵育1 h, PBS洗3次后用PBS配制的25 mg/L的FITC-Hoechst在室温下避光染色30 min, 用含50%甘油的磷酸盐缓冲液封片。FITC-Hoechst染色模式与顶体状态的关系定义为:顶体部全部染色者为顶体完整, 未发生顶体反应; 顶体部无荧光染色仅赤道板有荧光染色者为已发生顶体反应。
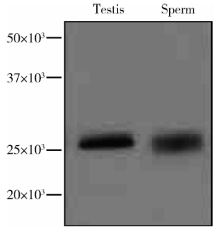
采用Western blot用CMTM2抗体对人睾丸及精子蛋白进行检测, CMTM2的表达结果显示, 在理论大小处均能获得单一清晰的条带, 表明抗体可以特异性地识别人睾丸及精子中的CMTM2, 证实了CMTM2在人睾丸及精子中均有表达(图2)。
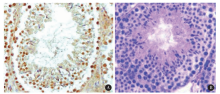
CMTM2免疫组织化学阳性反应呈棕黄色, 检测结果显示其在睾丸生精小管中广泛表达, 存在于各级生精细胞, 其阳性染色主要位于各级生精细胞的细胞膜(图3)。睾丸组织冰冻切片免疫荧光检测的结果与免疫组织化学一致, 进一步证实CMTM2存在于睾丸各级生精细胞的细胞膜(图4)。
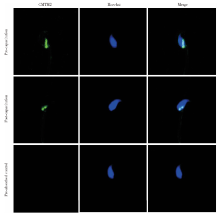
通过TRITC-CMTM2及FITC-Hoechst免疫荧光双染来检测CMTM2在人精子顶体反应前后的定位。Hoechst在顶体部染色完整表示未发生顶体反应, 顶体部无Hoechst荧光染色仅赤道板部有荧光染色表示已发生顶体反应。免疫荧光结果显示, 无论是顶体反应前的精子还是顶体反应后的精子, CMTM2均位于精子核后区附近, 并不随精子的顶体反应而改变(图5)。
目前对CMTM2这个跨膜蛋白尚缺乏了解, 此前有研究发现, CMTM2表达异常会造成生精过程障碍[4], 且随着生精障碍的加剧, CMTM2阳性细胞数目和mRNA表达水平显著下降, 在唯支持细胞综合征(seryoli cell only syndrome, SCOS)患者的睾丸组织中甚至无表达[4, 5, 6, 7]。上述证据都提示CMTM2可能在生精过程中发挥着一定的作用, 但具体机制尚不明确。
本研究对CMTM2在男性生殖系统的表达及定位进行了研究, 以成年生殖能力正常的男性睾丸及精子作为研究对象, 运用Western blot、免疫组织化学及免疫荧光检测等方法在蛋白水平较全面地检测了CMTM2在人睾丸及精子上的表达。Western blot检测证实了CMTM2在人睾丸和精子中均有表达, 人睾丸组织石蜡切片免疫组织化学及冰冻切片免疫荧光检测均显示CMTM2在生精上皮细胞内广泛表达, 并且表达在各级生精细胞的细胞膜上, 这与此前的定位研究类似[1, 2]。
CMTM2蛋白序列中含有MARVEL结构域, 而含有MARVEL的蛋白如MAL、physin和occludin家族等具有含胆固醇膜的定位事件、构成转运囊泡载体的功能[8], 因此, 人们认为CMTM2能够调节应激细胞中的囊泡转运, 有利于膜翻转, 并减少不必要的分泌, 认为CMTM2有可能参与膜运输, 如细胞间运输、分泌及小泡转运等过程[9]。CMTM2在生殖细胞的表达模式提示其可能参与生精小管中细胞间的相互作用, 如生精细胞间的信号传递、Sertoli细胞对生精细胞的营养支持等, 从而影响睾丸中精子的发生[10, 11, 12]。
本研究还检测了CMTM2在人精子的亚细胞定位, 结果显示无论对于顶体反应前的精子还是顶体反应后的精子, CMTM2在精子上的定位并不随精子的顶体反应而改变, 即顶体反应后精卵质膜融合时CMTM2仍位于核后区附近, 而此部位正是精子质膜与卵子质膜相互融合的部位[13, 14]。哺乳动物的受精是由一系列精卵质膜的相互作用而触发的, 这些相互作用的基本过程包括精卵识别、精卵结合和精卵质膜融合, 其中精卵质膜融合是受精过程最为关键的步骤[15, 16, 17]。精卵质膜融合是一个高度程序化的过程, 完成顶体反应是精子发生质膜融合的必要前提。
目前的研究已发现了一系列与精卵融合相关的蛋白, 而在本研究中CMTM2与NYD-SP8具有相似的精子亚细胞定位, 因此本研究推测CMTM2可能是一个受精相关蛋白, 在精卵质膜融合过程中发挥功能[18, 19]。
CMTM2在人睾丸及精子上的表达定位和特点, 提示其可能直接或间接影响精子的发生、发育和成熟, 并进一步影响精子与卵细胞的相互作用、相互识别和融合, 但要完全阐明CMTM2在男性生殖中的作用及其相关机制仍需进一步的研究。为探讨CMTM2在受精过程中所起的作用, 本研究下一步将通过一系列体外受精实验(如人精子穿去透明带仓鼠卵实验等)来对其功能进行深入研究, 以探寻CMTM2影响精卵融合的直接证据。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|