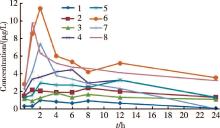
目的 他克莫司是一种新型钙调磷酸酶抑制剂,目前被广泛应用于成人肝或肾移植术后,也被逐渐广泛应用于肾病综合征患儿。他克莫司缓释胶囊是每日单次口服的缓释剂型,本研究目的是初步探讨他克莫司缓释剂治疗儿童原发性肾病综合征的药代动力学特征。方法 8例受试者系北京大学第一医院2011年6—8月原发性肾病综合征患儿。晨起单次口服不同剂量他克莫司缓释胶囊,给药剂量分别为0.02 mg/kg( n=2)、0.05 mg/kg( n=2)、0.10 mg/kg( n=4),在服药前及服药后1、2、4、6、8、10、12 h分别取静脉血1~2 mL,受试者不用影响他克莫司浓度的其他药物、食物及饮料。采用酶放大免疫分析法,测定他克莫司血药浓度,以Phoenix计算其药代动力学参数。结果 药代动力学数据采用非房室模型分析。3个剂量组(0.02 mg/kg,0.05 mg/kg和0.10 mg/kg)药代动力学参数如下:血药峰浓度分别为(1.7±1.0) μg/L,(3.1±1.9) μg/L,(8.0±3.5) μg/L;药物浓度-时间曲线下面积分别为(47.2±47.1) h·μg/L,(84.0±13.1) h·μg/L,(175.6±107.1) h·μg/L;表观清除率分别为(0.8±0.9) L/(h·kg),(0.4±0.1) L/(h·kg),(1.9±1.3) L/(h·kg);经剂量归一化的表观分布容积分别为(7.0±3.4) L/kg,(12.4±8.4) L/kg,(73.6±68.6) L/kg。0.05 mg/kg剂量组经剂量归一化的血药峰浓度和经剂量归一化的药物浓度-时间曲线下面积的平均值均高于0.02 mg/kg及0.10 mg/kg剂量组。3个剂量组的药物浓度-时间曲线均呈现2次高峰,第一次高峰出现在服药后约2 h,服药后约12 h出现次级高峰;0.10 mg/kg剂量组药物浓度出现两次峰值的现象较0.02 mg/kg及0.05 mg/kg剂量组更显著。结论 他克莫司缓释剂治疗原发性肾病综合征患儿的药代动力学存在个体间差异,本研究初步探讨了他克莫司缓释剂治疗儿童原发性肾病综合征的药代动力学特征,为后续大样本的研究提供了参考依据。
Objective: Tacrolimus prolonged-release(PR) formulation is a new once-daily formulation of the calcineurin inhibitor tacrolimus, which is currently used in adult liver or kidney transplant patients,and is also gradually widely used in children with nephrotic syndrome.The present study was undertaken to preliminarily investigate the pharmacokinetic characteristics of tacrolimus PR in pediatric nephrotic syndrome recipients.Methods: This single-center open-label prospective study was performed in pediatric nephrotic syndrome recipients. Pharmacokinetic samples were collected from eight pediatric subjects with nephrotic syndrome from Department of Pediatric Nephrology in Peking University First Hospital between June and August 2011. They followed administration of single oral doses of tacrolimus PR formulation at 0.02 mg/kg ( n=2), 0.05 mg/kg ( n=2) and 0.10 mg/kg ( n=4). Blood samples were taken before the dose and 1, 2, 4, 6, 8, 10, 12 and 24 h after drug intake. No other medicines or interacting food or drinks were taken during the study period. Blood concentrations were measured using an enzyme multiplied immunoassay technique. Pharmacokinetic analysis was performed using WinNolin Phoenix software Version 6.0(Pharsight, Cary, NC,USA).Results: The pharmacokinetic data were best described by a non-compartment model. Pharmacokinetic parameters of tacrolimus PR formulation in the 3 ascending doses groups (0.02 mg/kg,0.05 mg/kg and 0.10 mg/kg) were as follows: the maximum drug concentrations ( Cmax/ D) were (1.7±1.0) μg/L, (3.1±1.9) μg/L, (8.0±3.5) μg/L, respectively;Areas under the drug concentration-time curve(AU0-∞/ D) were (47.2±47.1) h·μg/L, (84.0±13.1) h·μg/L, (175.6±107.1) h·μg/L, respectively; Oral clearance rates were (0.8±0.9) L/(h·kg), (0.4±0.1) L/(h·kg), (1.9±1.3) L/(h·kg), respectively; Body weight normalized distribution volumes were (7.0±3.4) L/kg, (12.4±8.4) L/kg and (73.6±68.6) L/kg, respectively. Both mean Cmax normalized level for the administered dose( Cmax/ D) and mean AU0-∞ normalized level for the administered dose (AU0-∞/ D) were higher in the 0.05 mg/kg dosage group than in the 0.02 and 0.10 mg/kg dosage group. There were two peaks in the drug concentrations in every dose group;a primary peak appeared at the end of about 2 h followed by a small secondary peak at h 12, which was more noticeable in the 0.10 mg/kg dose group than in the two lower dosages.Conclusion: The pharmacokinetic characteristics of tacrolimus PR formulation were initially explored in pediatric patients with nephritic syndrome. The data presented form a basis for subsequent larger scale studies on pharmacokinetics of tacrolimus PR formulation in nephritic syndrome children.
Tacrolimus is a well-established immunosuppressant for the prevention and treatment of allograft rejection in solid organ transplantation, and forms the basis of immunosuppressive regimens in pediatric and adult patients[1]. Despite its success in ensuring graft survi-val, the therapeutic use of tacrolimus is complicated by its narrow therapeutic indexes of 5 to 15 μ g/L, wide intra- and inter-patient variability and the risk of interactions with concurrently used medications[2]. Trough tacrolimus blood concentrations have been shown to be related to clinical endpoints. Over immunosuppression with tacrolimus increases the incidence of infections and cancer, as well as the risk of nephrotoxicity, neurotoxicity, and other adverse events. Conversely, under immunosuppression increases the risk of allograft rejection[3]. These factors make defining an optimal dose schedule for tacrolimus difficulty[4]. Correlations between tacrolimus dose regimen and blood concentrations are week. Clinical research in Chinese adult renal transplant recipients suggested that genetic variation in Cytochrome P450 3A4(CYP3A4) and Cytochrome P450 3A5(CYP3A5), ABCB1 genotype, levels of hematocrit and concomitant use of drugs influenced the dose-adjusted tacrolimus concentration[5] .
Systemic exposure to tacrolimus (area under the curve, AUC) is a significant variable of efficacy. A study of 30 children reported that the tacrolimus AUC at a mean dosage of 0.12 mg/kg tacrolimus was 192 h· μ g/L[6]. Another group of workers reported that a mean dosage of 0.15 mg/kg resulted in a mean AUC of 197 h· μ g/L in 18 children 2 weeks after transplantation[7]. Tacrolimus prolonged-release(PR) formulation (Astellas, Levallois-Perret, France) is a new formulation, developed to provide once-daily dose while providing similar efficacy, tolerance and safety as the standard twice-daily formulation[8]. A study of 19 Canadian pediatric kidney transplant recipients suggested that the two formulations had bioequivalence after the conversion on a 1 ∶ 1 basis from standard twice-daily formulation to tacrolimus PR formulation[9].Another study of 133 Caucasian adult liver transplant recipients showed similar total AUC for both standard twice-daily formulation and tacrolimus PR formulation when targeting the same trough level[10].
Studies in adult kidney transplant recipients show different pharmacokinetic profiles in white populations than in African Americans and Asians[11, 12]. How-ever, published data for tacrolimus PR formulation in Chinese patients with chronic kidney disease patients are scant, particularly in children. Over the years it has been observed that a subgroup of Chinese children with kidney disease required a higher dose of tacrolimus to achieve the target therapeutic trough levels of 5 to 8 μ g/L. Abbreviated pharmacokinetic studies were therefore undertaken to determine optimal tacrolimus dose and frequency of administration. It is also important to establish the relationship between dose and trough concentrations, achieving the following once-daily dose with this prolonged-release formulation of tacrolimus. Here, a study is presented to explore the pharmacokinetic parameters after administration of a single oral dose of tacrolimus PR formulation to Chinese children with nephrotic syndrome.
This single-center open-label prospective study was performedfor pediatric nephrotic syndrome reci-pients. The study population comprised eight pediatric nephrotic syndrome recipients presenting at the Department of Pediatric Nephrology in Peking University First Hospital between June and August 2011.
All the subjects were separately administered a single oral doses of 0.02, 0.05 or 0.10 mg/kg tacrolimus PR formulation. The two steroid dependent patients were assigned into the 0.02 mg/kg group, and the two youngest steroid resistant patients into the 0.05 mg/kg group, and the four relative elder steroid resis-tant patients into the 0.10 mg/kg group. All the decisions were for interests of the patients. During the study, no severe adverse effect was observed in the patients. Blood samples were taken before the dose and 1, 2, 4, 6, 8, 10, 12 and 24 h after drug intake. The patients remained hospitalized during the period of blood sampling. No other medicines or interacting food or drinks were taken during the study period.
The study protocol was approved by the China State Food and Drug Administration(SFDA) and the Medical Ethics Committee of Peking University First Hospital. The study was conducted in accordance with the Declaration of Helsinki, and the data were collec-ted according to the principles of Good Clinical Practice. The childrens’ parents provided written informed consent prior to the patients being enrolled in the study.
Blood concentrations were measured using an enzyme multiplied immunoassay technique (Dada Be-hring Diagnostics, Milton Keynes, UK)[13]. This was a homogeneous enzyme immunoassay technique, based on a competition for antibody binding sites between tacrolimus in the sample and tacrolimus in the reagent, labeled with recombinant enzyme glucose-6-phosphate dehydrogenase[14]. In accordance with the manufac-turer’ s instructions, the lower limit of quantification was 2.0 μ g/L and the linearity ranged from 2.0 to 30 μ g/L.
Pharmacokinetic analysis was undertaken using the non-compartment modeling program WinNolin Phoenix software Version 6.0(Pharsight, Cary, NC, USA). Area under the drug concentration-time curve during the dose interval (AUC0-t) was determined using the linear trapezoidal rule. The constant was determined from regression analysis of concentration-time points in the terminal elimination phase. AUC0-t was added to the terminal area to obtain total AUC0-∞ . Maximum drug concentration (Cmax) and time to reach the maximum drug concentration (tmax) were the observed values. Terminal elimination half-life (t1/2) was calculated as 0.693/λ z, oral clearance (CL/F) as dose/AUC0-∞ , and volume of distribution by body surface area (V/F) as CL/(F· λ z).
Statistical analysis was performed using SPSS for Windows, version 16.0. Measurement data were mean ± standard deviation for statistical description if accorded with the normal distribution. Non-normal date were presented as median(min, max). Categorical data were presented by frequency and percentage.
Patient demographics and characteristics are shown in Table 1 and individual subject pharmacokinetic profiles 24 h after single oral dose of tacrolimus PR formulation are presented in Figure 1. The corresponding parameters at different dosages are shown in Table 2. Plasma concentration time curves for each dose level are shown in Figure 2. There were two peaks in drug concentrations; a primary peak appeared at the end of about 2 h followed by a small secondary peak at h 12 which was more noticeable in the 0.10 mg/kg dose group than in the two lower dosages.
In the 3 ascending dosage groups (0.02, 0.05 and 0.10 mg/kg), the mean Cmax normalized for the administered dose(Cmax/D) were 1.7, 2.1, 1.9 kg· μ g/(L· mg), respectively; Average AU
| Table 1 Demographic, laboratory, and renal function data |
| Table 2 Tacrolimus PR single-dose pharmacokinetic parameters by subject |
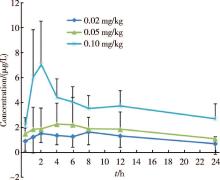 | Figure 2 Tacrolimus PR average blood concentrations curve with different dosage group for 0-24 hours after oral administration once |
| Table 3 Tacrolimus PR single-dose pharmacokinetic parameters by dosage group |
No adverse events were reported during the 7 days observation period and there were no cases of dose modification, interruption, or withdrawal from the study.
This is a pharmacokinetic study of prolonged-release formulation of tacrolimus in pediatric nephrotic syndrome recipients. The study population included two subjects with steroid-dependent primary nephritic syndrome, neither of whom was taking prednisone or methylprednisolone. We also wanted to see if the pharmacokinetics of tacrolimus was affected by disease type. Whereas the two steroid dependent patients did not need much dose, the two were brought into the lowest dosage group, which was also in the consideration of the morality and ethics. Maybe somebody would query the potential bias on the data, the patient’ s benefit was first.
It was found that the measurement of drug exposure per individual patient determined using the AUC de-pended on the dosage, suggesting that the higher group required a higher AUC than the lower group. However, no clear relationship was established when AUC was normalized to the administered dose (Table 3).
Half-life is a measurement of how rapidly drugs are cleared from the body, and is determined by the status of relevant drug-metabolizing enzymes and transporters. As shown in Table 3, the 0.02 mg/kg dose level exhibited a significantly faster tacrolimus clearance than that in the two higher dosage groups.
The present data for Cmax/D, AUC0-∞ /D and t1/2 are similar to those reported in a study comparing Hispanic and non-Hispanic children with kidney transplants, although CL/F in the present study is lower than that in the previously reported study [3].Multiple factors may be responsible for differences in long-term outcomes among children of different ethnic backgrounds, including biological differences in drug metabolism and pharmacokinetics. However, it has been reported that CL/F data are inconclusive for pediatric Chinese kidney disease recipients with some reports suggesting similar trends as in adults[15]. The mean CL/F across all doses of tacrolimus in the present study (Table 3) is in agreement with previously reported va-lues of 0.76 L/(h· kg) in 50 pediatric kidney transplant patients [16].
A study of 30 children reported that the tacrolimus AUC at a mean dosage of 0.12 mg/kg tacrolimus was 192 h· μ g/L [6]. Another group of workers reported that a mean dosage of 0.15 mg/kg resulted in a mean AUC of 197 h· μ g/L in 18 children 2 weeks after transplantation [7]. These values are higher than the mean AUC values obtained across all doses in our stu-dy. However, the mean doses in these two studies were also notably higher than those in our study.
A limited retrospective review of published tacrolimus pharmacokinetic profiles in Chinese pediatric recipients with kidney disease suggests that important dose-related differences may exist for various pharmacokinetic parameters[3]. However, in our study it was not possible to estimate these differences due to the small sample size and high degree of inter-individual variation. But despite the large interindividual variabi-lity in absolute values the overall drug concentration-time profile curves for tacrolimus PR formulation appear to be similar (Figure 1 and 2).
The pharmacokinetics parameters of tacrolimus are affected by various factors. Associated clinical covariates reported include genetic variation in CYP3A4 and CYP3A5, ABCB1 genotype and levels of hematocrit[5]. Ethnic variability in hepatic CYP3A activity or expression is inconsistent among different publications and is poorly characterized in the literature[17]. Stu-dies in adult kidney transplant recipients show different pharmacokinetic profiles in White subjects compared with African Americans and Asian subjects[3, 4]. This may be due to the higher expression of drug-metaboli-zing enzymes and/or transporters leading to faster tacrolimus clearance. However, one study[12] found no differences in intravenous tacrolimus pharmacokinetic profiles in healthy adult White, African American, and Hispanic volunteers. These observations may be attri-buted to ethnically associated differences in intestinal CYP3A and/or P-glycoprotein activity rather than to hepatic CYP3A activity. P-glycoprotein (P-gp) is the product of ABCB1 gene and acts as transmembrane efflux pump for many drugs[18]. It has been published in the literature that changes in the activity and levels of P-gp are one of the possible mechanisms of drug resis-tance[19].Significantly higher expression of ABCB1 and P-gp activity in T lymphocytes was detected in steroid-resistant nephrotic syndrome children compared with sensitive children[20].Corticosteroids are strong indu-cers of CYP3A4 and ABCB1, resulting in influences on trough concentrations of tacrolimus as well. Additionally, a study in pediatric liver recipients has showed that younger children need more tacrolimus than older children to reach the same tacrolimus target concentration but the reason is unknown. A wide range of age-related differences in drug disposition, such as CYP3A4/5 metabolism and P-gp transport, protein and erythrocyte binding was suggested[5].
A major limitation of this small retrospective ana-lysis was that it included only patients with problems achieving adequate target tacrolimus trough levels, and this selection bias limited the scope of our conclusions. In addition, due to the small sample size, this study was unable to consider other independent variables such as age. Given these limitations, our review stresses the need for a larger number of prospective studies in pediatric subjects in order to help us understand ethnic differences in children with nephrotic syndrome. This population, who often exhibit lower AUC at a given dose when adjusted for weight or surface area, and a larger volume of distribution, require a higher or more frequent dose. This highlights the need to perform larger scale pharmacokinetic studies in Chinese children with nephrotic syndrome who fail to achieve target tacrolimus trough levels.
Pharmacokinetics of tacrolimus PR formulation in pediatric nephrotic syndrome recipients showed inter-individual variation. Our data for Cmax/D, AUC0-∞ /D and t1/2 are similar to previously published findings but the values of CL/F were lower than those documented in the literature. Higher Cmax/D and AU
(Acknowledgments:We express our thanks to the children and their parents who participated in this study. We also acknowledge the support of the local clinical investigators and technicians who conducted the stu-dy. This work was not financially supported by institutional or pharmaceutical company grants.)
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|