目的 检测趋化因子配体19(C-C chemokine ligand 19, CCL19)在系统性红斑狼疮(systemic lupus erythematosus, SLE)患者血清中的表达,并分析其与SLE患者临床和实验室指标的关系,探讨CCL19在SLE发病机制中的可能作用。方法 采用酶联免疫吸附试验(enzyme linked immunosorbent assay, ELISA)法检测90例SLE患者(未接受过糖皮质激素和免疫抑制剂治疗的初治患者15例,曾治疗过的患者75例)和30名健康对照血清中CCL19的表达水平,分析SLE患者血清CCL19水平与临床特征和实验室指标的相关性。利用流式细胞术检测SLE患者B细胞及其亚群的比例,并进行血清CCL19水平与B细胞及其亚群比例的相关性分析。数据分析采用独立样本 t检验、配对 t检验、Pearson和Spearman相关分析。结果 (1)SLE初治患者和经治患者血清CCL19表达水平[分别为(596.25±409.19) ng/L和(422.90±395.84) ng/L]显著高于健康对照组[(157.79±125.23) ng/L, P均<0.001],初治患者组血清CCL19表达水平又高于经治患者组( P<0.05);(2)SLE患者血清CCL19表达水平与抗双链脱氧核糖核酸(double-stranded deoxyribonucleic acid, dsDNA)抗体、抗核小体抗体(anti-nucleosome antibody, AnuA)水平呈正相关(分别为 r=0.38, P=0.007; r=0.332, P=0.029),与免疫球蛋白IgA、IgG、IgM水平呈正相关(分别为 r=0.30, P=0.005; r=0.31, P=0.003; r=0.469, P=0.0001);(3)SLE有光过敏、关节炎和继发干燥综合征患者血清CCL19表达水平[分别为(562.25±399.12) ng/L、(565.6±435.24) ng/L和(694.9±531.02) ng/L]分别较无光过敏、无关节炎和未继发干燥综合征SLE患者高[分别为(394.7±281.42) ng/L、(385.90±325.33) ng/L和(424.8±305.46) ng/L], P均<0.05;(4)血清CCL19水平与外周血CD27-B 细胞和CD27-IgD-双阴性记忆性 B 细胞的比例呈正相关(分别为 r=0.519, P=0.007; r=0.461, P=0.018),与CD27+记忆性B细胞和CD27+IgD-转化后记忆性B细胞的比例呈负相关(分别为 r=-0.433, P=0.027; r=-0.616, P=0.001)。结论 SLE患者血清中高表达CCL19,与自身抗体的产生显著相关,CCL19可能通过影响B细胞亚群分布的内稳态参与SLE发病。
Objective: To detect the levels of serum C-C chemokine ligand 19 (CCL19) in patients with systemic lupus erythematosus (SLE) and to evaluate the correlation between CCL19 expression and clinical features and laboratory parameters, trying to reveal the possible role of CCL19 in the pathogenesis of systemic lupus erythematosus.Methods: The levels of serum CCL19 were measured by enzyme linked immunosorbent assay (ELISA) in 90 patients with SLE and 30 healthy controls. These SLE patients included 75 patients who received treatment with glucocorticoids and disease-modifying anti-rheumatic drug (DMARD) and 15 patients without therapy. The frequencies of peripheral blood B cells and the B cell subsets were assessed in the patients with SLE by flow cytometry. The correlation between the clinical data, laboratory parameters, B cell subset frequencies and serum CCL19 levels were analyzed. Indepen-dent samples t test, paired t test, Pearson and Spearman correlation were used for statistical analyses.Results: The levels of CCL19 were markedly higher in the SLE patients without therapy and the patients with therapy than in the health controls[(596.25±409.19) ng/L and (422.90±395.84) ng/L vs. (157.79±125.23) ng/L, all P<0.001]. Serum CCL19 levels in the SLE patients without therapy were higher than the SLE patients who accepted glucocorticoids and DMARD treatment ( P<0.05). The levels of serum CCL19 were positively correlated with anti-double stranded deoxyribonucleic acid (dsDNA), anti-nucleosome antibody (AnuA), IgA, IgG and IgM ( r=0.38, P=0.007; r=0.332, P=0.029; r=0.519, P=0.007; r=0.461, P=0.018, respectively). Serum CCL19 levels in the SLE patients with photosensitivity, arthritis and secondary Sjögren’s syndrome were higher than the SLE patients without photosensitivity, arthritis and secondary Sjögren’s syndrome, respectively [(562.25±399.12) ng/L, (565.6±435.24) ng/L and (694.9±531.02) ng/L vs. (394.7±281.42) ng/L, (385.90±325.33) ng/L and (424.8±305.46) ng/L, all P<0.05]. The levels of serum CCL19 were positively correlated with the percentage of CD27-B cells and CD27-IgD-double-negative memory B cells ( r=0.519, P=0.007; r=0.461, P=0.018, respectively). However, the levels of serum CCL19 were negatively correlated with the percentage of CD27+ memory B cells and CD27+IgD- switched memory B cells ( r=-0.433, P=0.027; r=-0.616, P=0.001, respectively).Conclusion: The increased serum CCL19 levels in SLE patients were associated with the production of autoantibodies, and CCL19 might be involved in the pathogenesis of SLE by disturbing the homeostasis of B cell subsets.
系统性红斑狼疮(systemic lupus erythematosus, SLE)是一种临床常见的、经典的、累及多系统的自身免疫性疾病, 其病因及发病机制仍未完全明确。近年来有关B淋巴细胞过度活化在SLE诱导发病和维持中的作用逐步得到重视, 其致病的可能机制包括自身反应性B细胞产生多重自身抗体、B细胞抗原提呈激活T细胞并促进其分化以及B细胞本身产生多种炎性细胞因子等[1, 2]。临床上B细胞靶向治疗的成功也突显出B细胞在SLE发病机制的重要作用[3]。
趋化因子配体19(C-C chemokine ligand 19, CCL19)结合趋化因子受体7(C-C chemokinreceptor 7, CCR7)后, 可在炎症及免疫反应中发挥重要作用[4]。研究显示, B淋巴细胞是表达CCR7的主要细胞之一[5]。然而, 在SLE患者血清中CCL19表达是否异常, 是否影响B细胞的免疫异常并参与SLE发病, 目前尚无相关报道。本研究通过检测SLE患者及健康者血清CCL19表达水平, 分析血清CCL19与SLE临床和实验室指标及外周血B细胞亚群的关系, 初步探讨CCL19在B细胞免疫异常和SLE发病过程中的作用及其临床意义。
从北京大学人民医院风湿免疫科2012年2月至2015年8月的门诊及住院患者中, 随机选取SLE患者90例, 其中女性78例, 男性12例, 年龄在15~76岁, 平均年龄(34.5± 12.1)岁, 均符合1997年美国风湿病学会(American College of Rheumatology, ACR)修订的SLE分类标准[6]。患者中, 未接受过糖皮质激素和免疫抑制剂治疗的初治患者15例, 曾治疗过的患者75例。另选取30例年龄和性别匹配的健康对照者(healthy controls, HC), 其中女性26例, 男性4例, 年龄24~56岁, 平均年龄(39.1± 15.2)岁。本研究经北京大学人民医院伦理委员会批准, 所有研究对象均签署知情同意书。
主要试剂及仪器:CCL19 Duo-set 酶联免疫吸附试验(enzyme linked immunosorbent assay, ELISA)试剂盒购自美国R& D公司; 酶标仪购自美国Bio-Rad公司; 流式抗体小鼠抗人CD19-APC-Cy7和IgD-FITC购自美国Biolegend公司, CD20-PE-Cy7、CD24-PE及CD27-APC购自美国eBioscience公司; 红细胞裂解液购自美国BD公司; 流式细胞仪(型号BD FACS AriaⅡ )购自美国BD公司。
血清趋化因子CCL19检测:将收集的血清以1:10 进行稀释, 采用ELISA法检测血清CCL19的浓度, 操作严格按照说明书进行。
外周血B细胞及其亚群的检测:采集新鲜的乙二胺四乙酸(ethylene diamine tetraacetic acid, EDTA)抗凝血100 μ L, 加入CD19-APC-Cy7、CD20-PE-Cy7、CD24-PE、CD27-APC及IgD-FITC流式抗体各5 μ L, 混匀后室温避光孵育30 min; 加入1× 红细胞裂解液2 mL, 振荡混匀室温避光孵育10 min; 1 600 r/min、离心5 min, 弃去上清液; 再将细胞沉淀用2 mL PBS重悬、离心, 洗涤一遍, 弃去上清液; 最后以200 μ L 2%(体积分数)多聚甲醛液重悬上机检测。检测过程中, 根据设门策略分别检测出CD19+ B细胞占淋巴细胞的百分率, CD19+CD27+记忆性B细胞、CD19+CD27-B细胞、CD19+CD27-IgD-双阴性记忆性B细胞、CD19+CD27-IgD+幼稚B细胞、CD19+CD27+IgD+未转化记忆性B细胞、CD19+CD27+IgD-转化后记忆性B细胞、CD19+CD27+CD20-浆细胞和CD19+CD24+CD27+B10细胞占B细胞的百分率。具体设门策略见图1, 为1例SLE患者B细胞及其亚群流式检测图。
临床及实验室资料采集:收集SLE患者详细的临床资料, 包括有无发热(发热定义为体温> 38 ℃, 且排除感染)、蝶形红斑、口腔溃疡、光过敏、浆膜炎(包括胸膜炎或心包炎)、贫血、脱发、神经精神性狼疮、继发干燥综合征等; 实验室指标包括血尿常规、24 h尿蛋白(> 0.5 g/24 h为蛋白尿)、红细胞沉降率、C反应蛋白、血清补体(C3、C4)、免疫球蛋白(IgA、IgG、IgM)、抗双链脱氧核糖核酸(double-stranded deoxyribonucleic acid, dsDNA)抗体、抗核小体抗体(anti-nucleosome antibody, AnuA)等自身抗体。所有临床资料及实验室数据采集时间均与此次血清收集时间一致。
疾病活动度评估:收集的临床及实验室资料按Gladman等[7]提出的SLE疾病活动度评分(SLE disease activity index, SLEDAI) 对SLE患者进行评分。
采用SPSS 20.0软件(IBM, 美国)对数据进行分析。非正态分布的资料采用中位数(四分位间距) [M(P25, P75)]表示, 两组间比较根据数据是否符合正态分布, 分别采用独立样本t检验和非参数独立样本t检验; 相关性分析根据数据是否符合正态分布, 分别采用Pearson和Spearman相关分析, P< 0.05为差异有统计学意义。
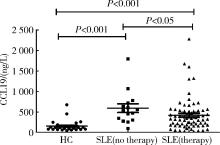
血清CCL19在初治SLE患者组和经治SLE患者组的表达水平分别为(596.25± 409.19)ng/L和(422.90± 395.84)ng/L, 均较健康对照组(157.79± 125.23)ng/L显著增高(分别为Z=-4.628, P< 0.001和Z=-4.685, P< 0.001); 初治SLE患者组血清CCL19表达水平又高于经治SLE患者组(Z=-2.236, P< 0.05, 图2)。
SLE患者血清CCL19水平与SLEDAI、C3、C4病情活动指标和白细胞、血小板减低无显著相关性; 但与抗dsDNA抗体、AnuA水平呈正相关, 与免疫球蛋白IgA、IgG、IgM水平显著正相关(图3)。
在SLE的临床表现中光过敏、关节炎和继发干燥综合征患者血清CCL19表达水平较SLE无光过敏、无关节炎和未继发干燥综合征患者高, 未发现血清CCL19表达水平在其他临床指标的阳性组与阴性组间的差异有统计学意义(表1)。
| 表1 SLE患者血清CCL19表达水平与各临床指标的关系 Table 1 Associations between serum CCL19 levels and clinical features of SLE |
本研究运用流式细胞术测定了26例SLE患者的外周血B细胞及其亚群比例, 并与患者血清CCL19水平进行了相关分析。
研究结果发现, 血清CCL19水平与外周血CD19+CD27-B 细胞和CD19+CD27-IgD-双阴性记忆性B 细胞呈正相关, 与CD19+CD27+记忆性B细胞和CD19+CD27+IgD-转化后记忆性 B细胞呈负相关, 未发现与总B细胞、CD19+CD27+CD20-浆细胞、CD19+ CD24+CD27+B10细胞及其他B细胞亚群有显著相关性(表2)。
| 表2 CCL19与B细胞及其亚群的相关性分析 Table 2 Correlations between serum CCL19 levels and B cell subsets |
SLE的主要免疫学特征是多克隆B淋巴细胞高度活化、大量自身抗体产生和免疫球蛋白增多[8]。B细胞免疫异常被视为SLE发病的中心环节, 有学者甚至认为SLE是一种B细胞介导的疾病[9]。近年来研究显示, SLE患者外周血B细胞亚群改变(如自身反应性B细胞比例升高而具有免疫调节能力的B细胞数量减少且功能降低)可能在SLE发病机制中发挥重要作用[10, 11, 12, 13, 14], 因此, 进一步探讨导致B细胞亚群改变的机制将有助于对SLE发病机制的认识和为靶向治疗提供理论依据。
趋化因子是一类结构相似且相对分子质量在8 000~16 000的小分子多肽, 与细胞表面相应受体结合后发挥生物学效应。研究显示, SLE患者血清中存在多种趋化因子高表达, 且证实趋化因子及其受体与SLE的免疫病理机制相关[15, 16]。CCL19是CC(C-C motif)家族成员, 与B细胞表面的相应受体CCR7特异性结合后在免疫反应中发挥重要作用。国内学者证实, CCL19在B淋巴细胞白血病的发病机制中起重要作用[17]。最近多项研究表明, 趋化因子CCL19及其受体CCR7可能参与了自身免疫病的发生。Sellam等[18]发现类风湿关节炎(rheumatoid arthritis, RA)患者血清 CCL19水平与外周血B细胞亚群分布相关, 且可作为预测利妥昔单抗临床反应的生物标记。Mathes 等[19]研究显示, 皮肤组织CCL19的表达与系统性硬化(systemic sclerosis, SSc)的皮肤血管炎相关, 机制可能为CCL19向局部组织募集免疫细胞和炎症细胞。
本研究发现SLE患者血清CCL19表达明显高于健康对照者, 且初治患者CCL19表达水平高于经治患者, 增高的血清CCL19浓度与抗dsDNA抗体、AnuA及免疫球蛋白水平呈正相关, 这提示SLE患者血清CCL19表达可能与B细胞活化以及产生自身抗体相关, 经糖皮质激素或免疫抑制剂治疗后血清CCL19水平降低。既往已证实抗dsDNA抗体、AnuA与肾受累及疾病活动度相关, 然而本研究没有发现CCL19表达水平与SLE患者SLEDAI评分和尿蛋白阳性相关, SLE患者肾损伤及发病机制复杂, 同时趋化因子也是一个复杂的免疫调控网络, CCL19在SLE肾受累中的作用有待进一步研究。此外, 本研究比较了SLE患者各临床指标与血清CCL19表达的关系, 发现伴有光过敏、关节炎患者较无光过敏、关节炎患者血清CCL19的表达水平高, 这与前期CCL19在SSc的皮肤血管炎和RA的研究有相似之处, 提示CCL19可能参与了SLE患者光过敏和关节炎的发病, 其具体机制需要进一步研究。同时, 本研究发现SLE继发干燥综合征的患者血清CCL19表达水平高于未继发干燥综合征的SLE患者, 提示CCL19与SLE继发干燥综合征有关可能是因为CCL19高表达的患者其免疫球蛋白相对较高引起的。
为进一步阐明CCL19在SLE发病中的可能机制, 本研究分析了SLE患者血清CCL19水平与B细胞及其亚群的关系。CD27为传统记忆性B细胞的表面标记, 近年来研究证实部分CD27阴性的B细胞也是记忆性B细胞的重要亚群[20, 21]。根据CD27和IgD表面标记将B细胞划分为CD27-IgD+幼稚 B细胞、CD27+IgD+未转换记忆性 B 细胞、CD27+IgD-转换后记忆 B 性细胞和CD27-IgD-双阴性记忆性 B 细胞[22]。既往研究证实, SLE患者外周血B细胞亚群分布内稳态被打乱, CD27+记忆性B细胞占外周血B细胞比例下降, 而CD27-IgD-双阴性记忆性B 细胞在SLE患者中比例明显升高且和疾病活动度、抗dsDNA抗体以及其他SLE自身抗体相关[10, 11, 12, 21, 23]。本研究发现, SLE患者血清CCL19水平与CD27-IgD-双阴性记忆性B细胞和CD27-B细胞比例呈正相关, 这可部分解释血清CCL19与自身抗体和免疫球蛋白的相关性, 提示CCL19作为B细胞的趋化因子可能参与了扰乱B细胞亚群内稳态的病理过程。同时, 本研究发现血清CCL19水平与CD27+记忆B细胞和CD27+IgD-转化后记忆 B细胞呈负相关。B细胞在RA的发病机制中也起重要作用, 上述结果与CCL19在RA中的相关研究结果一致[18]。在RA患者中, 这一现象可以用Nanki等[24]研究来解释, 他们发现CCL19受体CCR7在CD27- B细胞的表达明显高于CD27+ B细胞。然而, 是否在SLE发病过程中存在相同的机制, 需要进一步研究证实。
综上所述, SLE患者血清中升高的CCL19是一个与抗dsDNA抗体、AnuA滴度和免疫球蛋白水平密切相关的趋化因子, 其表达水平的升高可以提示SLE患者存在B细胞功能活跃和B细胞亚群分布异常, 同时CCL19较高的患者可能提示发生光过敏、关节炎和继发干燥综合征的概率增加。未来进一步探究CCL19与SLE患者B细胞免疫异常的分子机制, 将为阐明SLE的发病机制以及分子治疗提供理论依据。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|