肾尤文氏肉瘤/原始神经外胚层肿瘤(Ewing’s sarcoma/primitive neuroectodermal tumor,EWS/PNET)是一种罕见的肾肿瘤,恶性程度高,好发于儿童和青少年。患者多无特征性临床表现,常以腹痛或肉眼血尿而就诊。EWS/PNET临床进展迅速,易早期出现远隔转移并导致死亡,预后差,因此,及时、准确地做出诊断尤为重要。患者一经确诊,需接受包括根治性手术切除、化学治疗及放射治疗在内的综合治疗。遗憾的是,EWS/PNET在超声声像图及其他影像学检查中并无特异性表现。目前,肾EWS/PNET的诊断主要依靠其典型的组织学特点和免疫组织化学分析结果,特征性染色体异常也可作为有力的诊断依据。免疫组织化学分析CD99强阳性是其基本特征,t(11;22)染色体异位发生于大约90%的EWS/PNET病例中。本文报道了1例右肾EWS/PNET患者,患者为青年女性,以突发右侧腹痛为主要临床表现。超声检查时发现右肾下极可见一大的混合回声包块,对集合系统造成挤压导致肾上极扩张、积水。彩色多普勒检测到肿瘤内部存在动脉样血流频谱。患者接受了右肾肿瘤根治性切除术,并同时行右肾静脉和下腔静脉癌栓切除术。术后影像学检查提示,肿瘤未累及其他组织和器官。患者随后接受了共计6个周期的化学治疗。通过该肿瘤的特征性组织学特点和免疫组织化学结果,最终确诊为EWS/PNET。患者目前随访5年无复发征象。EWS/PNET的声像图表现虽无特异性,但超声检查仍是肿瘤初始评估的主要方法,超声引导下细针穿刺活检也具有重要的术前诊断价值。
SUMMARY Ewing’s sarcoma/primitive neuroectodermal tumor (EWS/PNET) in the kidney is a rare but high-grade malignant tumor that affects predominantly elder children and adolescents. Patients mostly present with nonspecific symptoms such as abdominal pain and gross hematuria. Since EWS/PNET has a rapid clinical progression with early metastasis and death, it is essential to make an accurate and early diagnosis. Once diagnosed, multimodality treatment, including radical surgery combined with adjuvant chemotherapy, and radiotherapy if necessary, is recommended. Unfortunately, there are no characteristic signsthat have been described in ultrasonography or any other imaging modalities so far. The diagnosis of EWS/PNET is now based on a classical histological and immunohistochemical investigation complemented by a demonstration of specific chromosomal changes. Strong immunoreactivity to CD99 is ubiquitous, and t(11;22) translocation is seen in approximately 90% of EWS/PNET. Herein, we report a patient with such condition. The patient was a young woman, and she presented with sudden right flank pain clinically. Ultrasonography revealed a large heterogeneous mass in the lower pole of her right kidney. The tumor compressed the renal pelvis and led to upper pole caliectasis. Color Doppler demonstrated blood flow with a pulsatile arterialized waveform within the mass. The patient received radical nephrectomy with right renal vein and vena cava thrombectomy. A search for other sites of tumor involvement yielded negative results. And six cycles of chemotherapy were sequentially performed. The diagnosis of EWS/PNET was confirmed based on primitive small round cell histology and characteristic immunohistochemical results. She was still alive with no evidence of recurrence five years after initial diagnosis. We would like to point out that ultrasound is still a useful method for initial assessment, and ultrasound-guided fine needle aspiration may play an important role in determining preoperative diagnosis.
Ewing’ s sarcoma (EWS) and primitive neuroectodermal tumor (PNET) are believed to share the same stem-cell precursor and have similar histologic and cytogenetic characteristics[1]. They occur most commonly in bone. Ewing’ s sarcoma/primitive neuroectodermal tumor (EWS/PNET) in genitourinary system is extraordinarily rare. Seemayer, et al.[2] initially described this entity in 1975. It predominantly affects elder children and adolescents, with an aggressive cli-nical course towards early metastasis and death. Thus, early diagnosis of renal EWS/PNET becomes quite important. However, its ultrasonographic manifestation has not been well characterized. Here we report a case of EWS/PNET in the right kidney to discuss the ultrasonographic features of this disease.
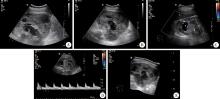
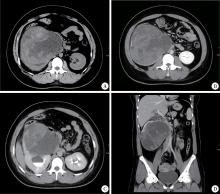
A 24-year-old woman presented with sudden right flank pain for two days. She reported no fever, gross hematuria, or weight loss. She had no other significant medial history. At physical examination, a large and firm mass was palpated in the right abdomen. On gray-scale ultrasound images, the right kidney was obviously enlarged and lack of reniform shape. A well-defined large heterogeneous mass measuring 15 cm× 14 cm was found in the lower pole of the kidney. It compressed the renal pelvis and led to upper pole caliectasis. Color Doppler demonstrated blood flow with a pulsatile arte-rialized waveform within the mass (Figure 1). A contrast-enhanced computed tomography (CT) of the abdomen was then performed revealing a 16 cm× 15 cm× 14 cm heterogeneous multi-septated right renal mass with tumor thrombus in the associated renal vein and inferior vena cava (Figure 2). Renal dynamic imaging indicated the patient’ s right renal function was moderately damaged. In December 2010, the patient underwent right radical nephrectomy with a presumptive diagnosis of renal malignant tumor. The right renal vein and vena cava thrombectomy were performed at the same time.
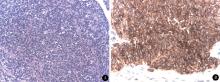
On gross examination, the tumor measured 13 cm× 10 cm× 9 cm, and it was well demarcated from the rest of the kidney. The cut surface was tan-yellow in color with multiple hemorrhage and necrosis. The perinephric fat, the Gerota’ s fascia, the right adrenal gland, and lymph nodes were free of tumor invasion. Histologic examination showed a small round cell tumor in hematoxylin-eosin (HE) staining. These tumor cells were arranged in a solid pattern and had a uniform dense, round to oval nuclei and scanty cytoplasm (Fi-gure 3). At places rosette-like structures were identified. Results of the immunohistochemical study were: CD99 (++), synaptophysin (-), chromogranin-A (-), CD10 (-), cytokeratin (-). Based on primitive small round cell histology and characteristic immunohistochemical profile, the tumor was diagnosed as renal EWS/PNET.
A search for other sites of tumor involvement, including chest CT scan, magnetic resonance imaging (MRI) of brain, and whole body bone scan, yielded negative results. Three cycles of chemotherapy with vincristine, adriamycin and cyclophosphamide, and three cycles of chemotherapy with ifosfamide and etoposide were sequentially performed. The patient was still alive with no evidence of recurrence five years after initial diagnosis.
EWS/PNET is the second most common malignant bone tumor in children and adolescents[3]. However, primary EWS/PNET of the kidney is extremely rare.
Since 1990s, a few cases of renal EWS/PNET have been reported in literature. These patients mostly presented with nonspecific symptoms such as abdominal pain and gross hematuria. No characteristic signs of renal EWS/PNET have been described in ultrasonography or any other imaging modalities. The diagnosis of EWS/PNET is now mainly based on histopathology, immunohistochemistry and cytogenetic analysis. Histologically, the tumor is characterized by sheets and nests of primitive small round cells with scanty cytoplasm and hyperchromatic nuclei. Tumor cells often tend to form perivascular rosettes and pseudorosettes. Immunohistochemical and cytogenetic analysis are crucial to confirm the diagnosis. Strong immunoreactivity to CD99 is ubiquitous[4, 5, 6], and t(11; 22) translocation is seen in approximately 90% of EWS/PNET[7, 8, 9]. Renal EWS/PNET is considered to have a poor prognosis with a tendency towards early metastasis[4]. Hence, multimodality therapy, including radical surgery combined with adjuvant chemotherapy, and radiotherapy if necessary, is now recommended worldwide for treatment.
The case we presented was a primary renal EWS/PNET in a young adult. She had a sudden right flank pain for two days. Ultrasonography and abdominal CT scan found a large heterogeneous right renal mass with tumor thrombus in the associated renal vein and inferior vena cava. The diagnosis of EWS/PNET was made based on the combination of histological features and immunohistochemical results. The patient received radical surgery and six cycles of chemotherapy in total. She was still alive with no evidence of recurrence five years after initial diagnosis.
Despite the technical advances in the field of imaging, no specific signs of renal EWS/PNET have been described in ultrasonography, and there is no literature focusing on the ultrasonography of this disease so far. To our knowledge, the sonographic features of EWS/PNET have a close relationship with the tumor pathology. Grossly, most EWS/PNETs are likely to be described as tan-yellow or gray-white lobulated solid tumors with multiple cystic components[5, 10, 11, 12, 13, 14, 15]. They are often identified as encapsulated masses with hemorrhagic and necrotic areas. Risi, et al.[16]reviewed 116 cases of EWS/PNET published from 1975 to 2012 and found that the median of maximum diameter was 13 cm. The reported ultrasonography findings[1, 12, 14, 17, 18, 19, 20] of renal EWS/PNET were consistent with their gross pathology. In most cases, the sonographic appearances of EWS/PNETs were well-defined solid masses. The echotexture of tumors were mostly isoechogenic or hyperechogenic in comparison with renal parenchyma. As the tumors grew, they tended to become more complex and heterogeneous as a result of necrosis and fibrosis. Cystic anechoic areas could be found within the masses, while calcifications were reported but uncommon. Renal pelvis and calyces could be dilated secondary to the obstruction caused by tumors. Color Doppler investigation mostly revealed internal vascularity with arterial waveform.
Given the uncharacteristic imaging appearance, we should put EWS/PNET into the differential diagnosis list when a large retroperitoneal mass with aggressive features is encountered. Other major differential considerations include Wilms tumor, neuroblastoma, malignant lymphoma, renal cell carcinoma, and metastatic sarcomatous lesions[7, 13, 18].
Recently, a handful of reports of primary renal EWS/PNET diagnosed with the help of ultrasound-guided fine needle aspiration (FNA) have been published[21, 22, 23, 24]. FNA has several advantages over the traditional open incisional biopsy. It has reduced risk of tumor cell contamination of the needle tract, as well as lower possibilities of morbidity and mortality. Besides, FNA is easy to learn by most physicians. Kumar reported a patient with a large renal tumor who received FNA under ultrasound guidance[24]. Relying on the FNA material, the EWS/PNET diagnosis was made preoperatively by cytological findings and subsequently confirmed by immunohistochemistry. Further more, Sanati, et al.[25] reported three cases of soft tissue EWS/PNET and demonstrated that FNA material can also provide a sufficient substrate for molecular genetic testing, including fluorescence in situ hybridization (FSH) and reverse transcription-polymerase chain reaction (RT-PCR). Molecular testing can enhance diagnostic certainty, especially when cytological or immunohistochemical features are atypical. All these suggest that ultrasound-guided FNA may play an important role in determining preoperative diagnosis.
In general, it is believed that an accurate and early diagnosis of renal EWS/PNET is important because of its high aggressiveness and poor prognosis. While rare, we should include EWS/PNET in the differential diagnosis of renal tumors, especially in young patients. Though ultrasonographic manifestation of EWS/PNET is nonspecific, ultrasound is still a useful method for initial tumor assessment, and ultrasound-guided fine needle aspiration may play an important role in determining preoperative diagnosis.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|