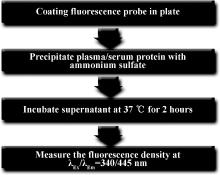
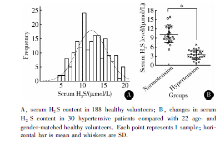
目的 在荧光探针的基础上建立一种简易的方法来检测血液中的硫化氢(hydrogen sulfide, H2S)。方法 将荧光探针包被到96孔板上,冷藏待用。用饱和硫酸铵沉淀血浆或血清中的蛋白,离心后所得的上清液分别加到含包被探针和不含包被探针的微孔中, 37 ℃避光孵育2 h,然后使用分光光度计(波长λEx/λEm 340/445 nm)读取微孔的荧光值,计算包被有探针和相应无探针微孔的荧光差值,根据标准曲线浓度计算出血液中H2S的浓度。结果 灵敏度和特异性测试表明,此方法检测灵敏度下限可以达到0.3 μmol/L,血液中其他成分甚至其他含硫活性成分和含硫氨基酸等对此方法影响甚微。应用此方法检测188名健康成年志愿者的血清H2S浓度[(12.1±3.5) μmol/L,95% CI: 4.6~19.8 μmol/L],所得结果呈正态分布(单样本K-S检验, P>0.1)。对30名高血压患者和22名相匹配的健康志愿者血清H2S浓度检测表明,前者H2S浓度低于后者[(3.52±1.49) μmol/L vs. (10.23±2.76) μmol/L],差异具有统计学意义(配对样本 t检验, t=9.937, P<0.001)。检测雄性Wistar大鼠血清[(19.66±2.32) μmol/L]和血浆[(18.67±2.07) μmol/L]以及动脉血[(19.34±0.51) μmol/L]和静脉血[(18.99±0.50) μmol/L]中的H2S浓度,结果表明雄性Wistar大鼠血清和血浆以及动脉血和静脉血两者差异都没有统计学意义(重复测量的方差分析, P=0.38)。样品的重复性检测稳定性较好(两因素方差分析, P>0.05)。结论 此方法对检测血液中的H2S具有简单、高灵敏度、特异性和可重复性等优点,出结果快,可适用于大样本的高通量检测,满足实验室基础研究及临床一次性大样本H2S浓度检测的需求。
Objective: The hydrogen sulfide (H2S) role in pathogenesis of various diseases were wildly addressed in recent decade. The circulatory (plasma or serum) and biological fluid H2S measurement is still an enormous issues due to the technical limitation. This paper aimed to develop a novel measurement method based on fluorescence probe.Methods: Firstly, 20 μL ethanol was used to dissolve 100 pmol fluorescence probe, then added in a 96-well plate. An equal volume of ethanol was also added to the blank well of the plate. The plate was placed in a dark room for about 1 h until the fluorescence probe was evenly coated in the 96-well microplate and dried. The plate was frozen at -20 ℃ for later use. Secondly, the plasma or serum sample was added with saturated ammonium sulfate buffer (pH 7.8) and then centrifuged to remove the proteins. The equal volume supernatant liquid was added to the probe-coated well and the probe-uncoated well. The plate was incubated in a dark environment at 37 ℃ for 2 h. Finally, after incubation, the fluorescence density was acquired at λEx/λEm 340/445 nm in a microplate reader. The differences of the fluorescence density values between the probe-coated well and probe-uncoated well were counted and H2S concentration of plasma/serum was calculated by standard curve with NaHS.Results: The method had high sensitivity (from 0.3 to 100 μmol/L) and specificity for measuring H2S as compared with other biologically relevant reactive sulfur species and sulfur-containing amino acid. Serum H2S concentrations were assayed in 188 health volunteers using this method [(12.1±3.5) μmol/L, 95% CI: 4.6-19.8 μmol/L], and the frequency distribution showed a normal tendency(one-sample Kolmogorov-Smirnov test, P>0.1). The serum H2S concentrations in 30 hypertension patients were decreased compared with 22 age- and gender-matched health individuals (paired-samples t test, t=9.937, P<0.001). There were no differences of H2S concentration in serum [(19.66±2.32) μmol/L] or plasma [(18.67±2.07) μmol/L], between the samples acquired from artery [(19.34±0.51) μmol/L] or vein [(18.99±0.50) μmol/L] of male Wistar rats (repeated measurement of ANOVA, P=0.38). One week frozen samples did not affect the detection. The values of the repeated measurement did not differ (two-way ANOVA, P>0.05).Conclusion: The present method is easily performed with high sensitivity, specificity and repeatability for circulatory H2S. It is also quick and may apply for large samples.
Hydrogen sulfide (H2S), as the third gasotransmitter, plays an essential role in physiological function and is involved in pathophysiological regulation in cardiovascular, nervous, respiratory, gastrointestinal and metabolic diseases[1]. However, the study of H2S in human diseases has not been fully investigated because of limitations in measurement methods for circulatory H2S. The range of circulatory H2S content is from 0.1 to 300 μ mol/L according to different disease models and measurement methods[2]. Four types of H2S, including free H2S, H2S anion (HS-), sulfide (S2-) and protein-bound sulfur, exist in blood circulation[3, 4].
All current detection methods are based on the measurement of free H2S, HS- or S2-. Methylene-blue-dependent colorimetric assay[5, 6] and sensitive sulfur electrode assays[7] are used to measure S2- level, which requires reversing H2S and HS- to S2-. The disadvantage of methylene blue assay is the inability to measure a low concentration of H2S, due to the buffer pH and disturbances of high protein content in biological samples. Using sensitive sulfur electrode assays, strong basic buffer might induce protein desulfurization, thereby resulting in artificially high H2S values. A polarographic sensor offers a selective H2S permeable membrane and increases the specificity for detection[8]. However, this sensor failed to detect blood or plasma sulfide in 6 species of vertebrates[9]; a high concentration of protein in blood reducing the membrane permeability may be the reason. The monobromobimane method based on the reaction of sulfide with 2 monobromobimanes to generate stable fluorescent sulfide dibimane is effective to measure H2S in plasma[10]. However, this method involves complicated procedures and is disturbed by polysulfides and a reducing substance (such as cysteine or glutathione). Gas chromatography[11, 12] is sensitive for detecting plasma H2S but it is not adaptable for large numbers of samples and its value depends on the experimental conditions (i.e., temperature, pH, pressure, chemical composition of gas phase).
Recently, H2S-specific and -sensitive fluore-scence probes have been designed and synthesized according to different chemical reactions and were sensitive and highly specific for measurement in live biological systems[3]. The major challenge of fluorescence probe-based measurement of H2S in plasma or serum is still to avoid the interference of other thoil species, such as glutathione and sulfur-containing amino acids, such as cysteine and a large component of protein in blood.
In the present study, this paper used a fluorescent probe (C-7Az) [13] to develop a method for detecting plasma and serum H2S in 96-well microplates.
The study was approved by the Ethics in Human Research Committee of Peking University.All animal procedures complied with the Animal Management Rule of the Ministry of Health, People’ s Republic of China (document No. 55, 2001) and the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication No. 85-23, updated 2011). The care and use of laboratory animals were approved by the Laboratory Animal Ethics Committee of Peking University. In the study of humams, all patients and volunteers were to give full and informed consent and the approvals by the Human Research Ethics Committee were obtained from The General Hospital of the People’ s Liberation Army (PLAGH).
Venous blood plasma samples were obtained from 188 healthy adult volunteers and 30 hypertensive patients recruited in Peking University Health Science Center. A total of 15 male Wistar rats (180-200 g) were supplied by the Animal Center, Peking University Health Science Center. Sodium hydrosulfide hydrate (NaHS), L-cysteine, homocysteine, L-methionine, bovine serum albumin (BSA), ammonium sulfate and L-glutathione reduced (GSH) were from Sigma-Aldrich, Inc., USA. COSTAR 96-well plates (product #3610) was from Corning Co., USA. The fluorescent probe (C-7Az) was a gift from Professor Tang Xin-jing (School of Pharmaceutical Sciences, Peking University).
The method for measuring plasma or serum H2S was as follows (summarized in Figure 1): (1) Fluorescence probe was coated in 96-well microplates. Then 100 pmol H2S-sensitive probe C-7Az in 20 μ L ethanol was added by using an interlaced model in the plate. An equal volume of ethanol was also added to the uncoated well. The plate was placed in a dark room for 1 h to dry and then stored at -20 ℃ after sealing the plate face. (2) Plasma or serum samples of 150 μ L were added in equal volume with saturated ammo-nium sulfate buffer (pH 7.8). The samples were centrifuged at 20 000× g at 4 ℃ for 15 min and the supernatant was transferred into a new tube. The supernatant was centrifuged again. (3) The supernatant (100 μ L) was added to the probe-coated well and another 100 μ L to an uncoated well. The plate was incubated in a dark environment at 37 ℃ for 2 h. (4) After incubation, the fluorescence density was acquired with excitation at λ Ex/λ Em=340/445 nm in a Fluo Star OPTIMA microplate reader (BMG Labtech Co.). The discrepant fluorescence density values between the coated-probe and blank well were measured, and the serum and plasma H2S concentration were measured according to a stan-dard curve with NaHS.
This study anesthetized 15 male Wistar rats with isoflurane. Arterial blood and venous blood was collected from the abdominal aorta or inferior caval vein, respectively. Plasma or serum from the same rat was prepared. Fresh samples were assayed for H2S, and another sample was quickly frozen at -80 ℃. After 1 week, these samples were assayed again. Sera from human volunteers were divided into 12 portions and frozen at -80 ℃. In the following week, one portion was assayed per day. H2S was also measured in the sera frozen for 1, 3 and 6 months.
The data were presented as mean± SD. The normality of data distribution of human serum H2S level was analyzed by one-sample Kolmogorov-Smirnov test. Differences between normotensive and hypertensive subjects were analyzed by paired-samples t test. Parametric t test was used to analyze the biological samples usually. While considering the data amomg the arte-rial, venous, plasma and serum groups were indepen-dent, repeated measurement of ANOVA was used. Among the groups of repeated measurement, two-way ANOVA was taken, then Student-Newman-Keuls test. P< 0.05 was considered statistically significant.
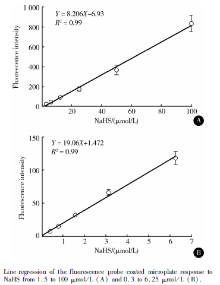
To investigate the sensitivity of the method for measuring H2S, different concentrations of NaHS were used as a standard H2S donor. In terms of fluroescence intensity, the probe responded to NaHS from 1.5 to 100 μ mol/L (most recent studies reported serum H2S level < 50 μ mol/L in humans[14, 15] ), Figure 2A. In investigating the detection limit, it also responded to NaHS from 0.31 to 6.25 μ mol/L (Figure 2B) but not < 0.3 μ mol/L NaHS (data not shown).
As H2S was a complicated component in blood, we needed to assess the specificity of the probe. Most inorganic ions did not previously respond to this probe[13]. Here, we found that Na2S, sulfur-containing amino acid (L-methionine, L-cysteine and L-homocysteine) and reduced GSH did not respond to the fluorescence probe, but BSA (50 g/L) increased the flurorescence density (Table 1). Therefore, the method had high specificity for H2S from plasma and serum.
| Table 1 Specificity of the fluorescence probe for H2S |
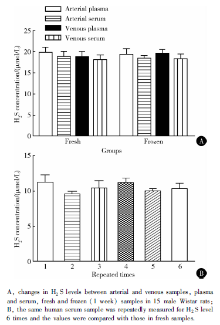
Because most clinical serum samples were collec-ted from the vein and frozen, we assessed the differences between the arterial samples and venous samples, and fresh and frozen samples. Arterial and venous plasma/serum samples did not differ in measurements (P=0.38, Figure 4A, Table 2). Freezing for 1 week did not affect the measurement values (P> 0.05, Figure 4A). However, freezing for 1, 3, and 6 months reduced serum H2S values about 30%, 60% and 80%, respectively (data not shown). Measurement of the human serum samples in successive 7 days did not affect H2S values (Figure 4B), which suggested the stability of this measurement method.
| Table 2 Descriptive statistics between arterial and venous plasma/serum samples in fresh and frozen groups |
In the present study, we developed a serum H2S detection method based on a specific fluorescence probe. The fluorescence probe had high sensitivity (lower limit 0.3 μ mol/L) and specificity for H2S. The method is effective for assessing changes in human serum H2S concentration and has high repeatability. It is quickly and easily performed and can be used for large samples.
The fluorescence probe C-7Az has high sensitivity and specificity for H2S as compared with other biologically relevant reactive sulfur species, reactive oxygen species and reactive nitrogen species[13]. As compared with other methods based on detecting S2- [3, 16], such as methylene blue assay, sulfur electrode assay, polarographic sensor and other fluorescence probes, C-7Az has higher selectivity for free H2S and HS-, a form of H2S in biological fluid. This probe does not respond to sulfur-containing amino acid and reduced GSH, so it avoids the detection interference of these physiological endogenous substances in blood. C-7Az also has large detection range, from 0.3 to 100 μ mol/L, which co-vers the range of H2S in blood according to recent stu-dies[3]. Especially, it also has good response at low concentration of H2S (Figure 2B), so it might work well with the reduced circulatory H2S level in some diseases.
High concentration of protein (5% albumin is similar to physiological content in blood) per se increased the fluorescence density with our probe. Because the excitation and emission spectrum of our probe fluorescence was located in the ultraviolet range, blood proteins might have background fluorescence at this spectrum. Also, protein-bound H2S might respond to the probe. Here, we used saturated ammonium sulfate buffer (pH 7.8) to remove plasma/serum protein, which reduced the interference of protein for measurement. Other substances in blood also had background fluorescence at an ultraviolet spectrum, so we mea-sured the changes in background fluorescence in prepared samples. In blank wells, the fluorescence density of the samples changed little during 12 h continuous measurements (data not shown). However, the fluorescence density in the coated-probe well and NaHS buffer increased by the time. The measurement of H2S in the samples did not change at different times in the standard curve. Thus, we selected 2 h incubation because the time was short and the content stable.
Using this new method, we assessed serum H2S level in 188 healthy volunteers and the method showed a good normal distribution and 28.9% coefficient of variant. We also found that serum H2S level was lower in hypertensive patients than age- and gender-matched healthy volunteers. Therefore, our method was effective for measuring H2S in human blood samples and might be responsive in different diseases. Some authors exogenously added S2- or NaHS directly to assess the operability of their methods[2, 13, 17]. However, exo-genous administration of H2S was quickly lost in biological fluid because of biological oxidation or utility[18]. So blood protein should be removed for detecting H2S concentration.
In most biological experiments or clinical studies, the blood samples are often frozen. Some variables also differ in arterial and venous blood. Using the new me-thod, we did not find differences in H2S level between plasma and serum or between arterial and venous samples. Short-term frozen storage (1 week) did not affect the measurement. However, with extended storage, H2S was lost in samples, which might limit its application in clinical study. Thus, we evaluated the repeatability of this method. The same human serum samples were used for 7 repeat measurements, and the acquired values for H2S content were stable (Figure 4B). Blood H2S measurement should be performed in 1 week with our method. The H2S level in different batch samples and changes in human diseases should be investigated.
In the present study, we developed a method for detecting blood H2S content in plates coated with a fluorescence probe. Plates with pre-coated probe might be easily used and avoid the measurement differences among repeatable experiments. Removing protein from plasma/serum guarantees the stability of the measurement. Because of the high sensitivity and specificity of our probe, we might assess true H2S content in blood. In fact, because of the efficiency of removing protein by saturated ammonium sulfate, we could not ensure that the protein had been clearly removed. Protein sulfhydration is a direct functional chemical modification by H2S in the body[19, 20]. H2S inhibits blood coagulation time and increases fibrin lysis[21], which may involve the sulfhydration of relative enzymes in the blood. Plasma protein sulfhydration should be taken into account when measuring H2S content.
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|