目的 探讨移植脂肪间充质干细胞(adipose tissue derived stem cells,ADSCs)对MRL/lpr小鼠的治疗作用及对MRL/lpr小鼠脾脏Th17/Treg细胞平衡的影响。方法 15只12周龄的雌性MRL/lpr小鼠采用随机数字表法分为ADSCs治疗组(ADSCs组)、对照组(control组)和环磷酰胺治疗组(cyclophosphamide,CTX组),每组5只。ADSCs组经尾静脉注射ADSCs,每次注射的细胞数为1×106/150 μL,每周1次,共注射8次;control组经尾静脉注射等体积磷酸盐缓冲液,每周1次,共注射8次;CTX组经尾静脉注射环磷酰胺,剂量为15 mg/kg(体重),每周1次,连用2次,休息2周后重复,共注射4次。留取治疗前和治疗后尿液检测24 h尿蛋白浓度。治疗8周后,处死MRL/Ipr小鼠,以流式细胞仪分析脾细胞Th17/Treg细胞比例变化。结果 (1)24 h尿蛋白浓度变化:治疗前,3组小鼠的24 h尿蛋白浓度差异无统计学意义( P>0.05);治疗4周后,ADSCs组和CTX组24 h尿蛋白浓度显著低于control组,差异有统计学意义[(5.02±1.61) g/L vs. (7.10±1.63) g/L、(4.90±0.71) g/L vs. (7.10±1.63) g/L, P<0.05],而且治疗时间越长,效果越明显,治疗8周后,ADSCs组和CTX组24 h尿蛋白浓度显著低于control组[(2.24±0.73) g/L vs. (10.36±1.64) g/L、(3.80±1.45) g/L vs. (10.36±1.64) g/L, P<0.01];ADSCs组和CTX组之间24 h尿蛋白浓度差异无统计学意义( P>0.05)。(2)脾脏Treg细胞占CD4+T细胞的百分率:ADSCs组和CTX组脾淋巴细胞中Treg细胞占CD4+T细胞的百分率高于control组,3组分别为13.62%±1.87%、14.14%±1.29%、0.71%±1.23%,但组间差异无统计学意义( P>0.05)。(3)脾脏Th17细胞占CD4+T细胞的百分率:ADSCs组和CTX组脾淋巴细胞中Th17细胞占CD4+T细胞的百分率显著低于control组,3组分别为1.43%±0.20%、1.63%±0.65%、6.37%±1.64%,差异有统计学意义( P<0.01)。结论 移植ADSCs能够减少MRL/lpr小鼠尿蛋白浓度,治疗时间越长,效果越显著。ADSCs能够降低MRL/lpr小鼠脾脏Th17细胞水平,提高Treg细胞水平,负向调节Th17/Treg细胞平衡,进而发挥抗炎和免疫调节的作用。
Objective: Preliminary study on therapeutic effects of adipose tissue derived stem cells (ADSCs) on MRL/lpr mice and the effect on imbalance of Th17/Treg.Methods: Fifteen 12-week-old MRL/lpr mice were randomly divided into 3 groups by using random number table, including ADSCs group, control group and cyclophosphamide (CTX) group, with 5 in each group. ADSCs group and control group were injected with 1×106ADSCs or phosphate buffered solution (PBS) via tail vein respectively, once a week, a total of eight times. CTX group was injected CTX at a dose of 15 mg/kg body weight, once a week for 2 weeks, and then repeated after 2 weeks’ rest, a total of four times. The 24-hour proteinuria was measured before and after treatment. All the mice were sacrificed after treatment for 8 weeks. Th17 cells and Treg cells in splenic were examined by flow cytometry.Results: (1) The 24-hour proteinuria in the three groups had no significant difference before treatment ( P>0.05). After therapy for 4 weeks, the 24-hour proteinuria in the ADSCs and CTX groups was much lower than those in control group, and the difference was significant [(5.02±1.61) g/L vs. (7.10±1.63) g/L, (4.90±0.71) g/L vs. (7.10±1.63) g/L, P<0.05], and the longer the duration of treatment (8 weeks), the more obvious effect [(2.24±0.73) g/L vs. (10.36±1.64) g/L, (3.80±1.45) g/L vs. (10.36±1.64) g/L, P<0.01]. There was no significant difference in 24-hour proteinuria between ADSCs group and CTX group ( P>0.05). (2) Percentage of Treg cells /CD4+T cells in the spleen lymphocytes: The percentages in ADSCs and CTX groups were higher than that in control group. The levels were 13.62%±1.87%, 14.14%±1.29%, 10.71%±1.23%, respectively, but there was no significant difference ( P>0.05). (3) Percentage of Th17 cells /CD4+T cells in the spleen lymphocytes: The percentages in ADSCs and CTX groups were significantly lower than that in control group. The levels were 1.43%±0.20%, 1.63%±0.65%, 6.37%±1.64%, respectively, with statistical significance ( P<0.01).Conclusion: Transplantation of ADSCs can reduce the 24-hour proteinuria in MRL/lpr mice. To prolong the time of treatment, the effect is more significant. Transplantation of ADSCs can up-regulate Treg cells and down-regulate Th17 cells. ADSCs have the ability to regulate the immune balance of Th17/Treg in MRL/lpr mice, suggesting that ADSCs play the role of anti-inflammatory and immune regulation by regulating the Treg and Th17 cells.
系统性红斑狼疮(systemic lupus erythematosus, SLE)是一种表现为多脏器损害的慢性系统性自身免疫性疾病, 以大量自身抗体产生、补体激活及免疫复合物沉积为主要特点。研究发现, 在SLE患者中存在T细胞的受损、Treg细胞和Th17细胞之间的平衡被破坏的现象[1, 2, 3]。目前, 多数SLE患者使用非特异性的免疫抑制药物以及糖皮质激素治疗, 但这些治疗方法效果仍不理想且有许多副作用。近些年, 采用异基因间充质干细胞(mesenchymal stem cells, MSCs)移植治疗难治性SLE患者取得了较好的疗效, 且具有安全、费用低的优点[4, 5]。脂肪间充质干细胞(adipose tissue derived stem cells, ADSCs)具有分化为多种细胞和免疫调节的能力。Ribeiro等[6]的研究发现, ADSCs比骨髓间充质干细胞和脐带间充质干细胞具有更强的抑制T细胞活化的能力, 因此, 本研究将ADSCs经尾静脉移植到系统性红斑狼疮模型MRL/lpr小鼠体内, 初步探讨移植ADSCs对MRL/lpr小鼠尿蛋白浓度以及脾脏Th17/Treg细胞平衡的影响。
SPF级MRL/lpr自发系统性红斑狼疮样模型小鼠15只, 雌性, 8~10周龄, 购自南京大学模式动物研究所, 适应性饲养2周后用于实验。小鼠采用随机数字表法分为3组, ADSCs治疗组(ADSCs组)、对照组(control组)和环磷酰胺治疗组(cyclophosphamide, CTX组), 每组5只, 由专人饲养。
DMEM/F12培养基和胎牛血清购自Gibco公司, 总蛋白定量测试盒(考马斯亮蓝法)购自生工生物工程(上海)股份有限公司, CD31-PE、CD44-FITC、CD45-FITC、CD90-PE流式抗体和Mouse Th17/Treg Phenotyping Kit购自BD公司。
取10只10周龄C57BL/6小鼠, 以10%(体积分数)水合氯醛3 mL/kg体重腹腔麻醉, 无菌条件下剪取腹股沟部皮下脂肪约1 g, 磷酸盐缓冲液(phosphate buffered solution, PBS)冲洗3次以去除肉眼可见的小血管和血细胞, 剪碎至1 mm3, 加入2倍体积的0.1%(质量分数)Ⅰ 型胶原酶, 于37 ℃温箱中振荡30 min, 加入等体积的含10%(体积分数)胎牛血清的DMEM/F12培养基, 用吸管吹打数次, 1 000 r/min离心10 min, 弃上清液, 再用含10%胎牛血清的DMEM/F12重悬细胞, 200目细胞筛过滤, 四格计数板计数后以1× 104/cm2的密度接种于50 mL 培养瓶中, 放置于37 ℃、5%(体积分数)CO2的孵箱内培养。24 h后首次换液, 之后每2~3天换液1次。光学倒置显微镜下观察细胞生长状况, 待细胞融合至瓶底80%以上时利用0.25%(质量分数)胰蛋白酶消化, 以体积比1 ∶ 3进行传代。收集第3代的ADSCs, 利用BD FACSCalibur流式细胞仪分别检测其表面抗原CD31、CD44、CD45和CD90。
检测尿蛋白浓度≥ 1 g/L时提示MRL/lpr小鼠发生自身免疫性疾病[7], ADSCs组经尾静脉注射ADSCs, 每次注射的细胞数为1× 106/150 μ L, 每周1次, control组尾静脉注射等体积PBS。CTX组经尾静脉注射CTX, 剂量为15 mg/kg(体重), 每周1次, 连用2次, 休息2周后重复。
分别于治疗第0、4、6、8周末将MRL/lpr小鼠放入代谢笼内, 用5 mL离心管收集小鼠24 h尿液, 置于-80 ℃冰箱中保存, 以考马斯亮蓝法集中检测尿蛋白浓度。
治疗8周后处死小鼠, 无菌摘取脾脏, 在70 μ mol/L的无菌尼龙网将脾脏剪碎、研磨, 收集到的脾细胞经红细胞裂解液裂解红细胞, 以2× 106/孔培养于12孔板中, 并加入50 μ g/L的PMA, 1 mg/L的离子霉素(ionomycin)以及莫能菌素(monensin), 置于37 ℃, 5% CO2培养箱中培养5 h, 收集细胞。加入抗CD4-PerCP-Cy5.5进行细胞表面染色, 温育15 min, 加1 mL固定液(fixation)温育15 min固定, 洗涤, 弃上清液。加入1 mL 破膜剂(permeabilization)破膜, 洗涤后加入抗小鼠IL-17-PE和抗小鼠Foxp3-APC单抗作胞内染色, 洗涤后上机检测。
用SPSS 17.0统计软件进行分析, 数据以均数± 标准差表示, 多个组间均数的比较采用LSD统计学方法分析, P< 0.05为差异有统计学意义。
治疗前, 3组小鼠的24 h尿蛋白浓度差异无统计学意义(P> 0.05); 治疗4周后, ADSCs组和CTX组 24 h尿蛋白浓度低于control组, 差异有统计学意义(P< 0.05), ADSCs组和CTX组之间24 h 尿蛋白浓度差异无统计学意义。治疗时间越长, 尿蛋白浓度下降越明显(P< 0.01)。具体结果见表1。
| 表1 各组小鼠尿蛋白浓度的变化 Table 1 Changes of urinary protein content of each group |
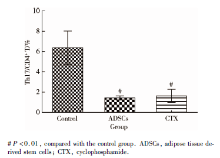
流式细胞检测结果显示, 与control组相比, ADSCs组和CTX组脾淋巴细胞中Treg细胞的比例稍高, Th17细胞的比例较低, 结果见图1、2。
ADSCs组和CTX组小鼠脾淋巴细胞中Treg细胞占CD4+T细胞的百分率高于control组, 分别为13.62%± 1.87%、14.14%± 1.29%、0.71%± 1.23%, 但组间差异无统计学意义(P> 0.05, 图3)。
间充质干细胞是干细胞家族的重要成员, 来源于发育早期的中胚层和外胚层。间充质干细胞移植在治疗血液系统疾病、心血管疾病、肝硬化、神经系统疾病和自身免疫性疾病等方面取得了重大突破。研究发现, 间充质干细胞可以通过抑制多种免疫相关细胞介导的免疫应答反应进行免疫调节, 另外, 其可抑制B细胞向浆细胞分化, 减少免疫球蛋白的生成, 理论上间充质干细胞的这种免疫调节作用可以很好地应用于自身免疫性疾病的治疗。有研究表明间充质干细胞有望成为新的类风湿关节炎的免疫治疗方法[8]。有研究使用异基因间充质干细胞移植治疗病情较重以及常规治疗不起作用的系统性红斑狼疮患者, 经过4年的研究发现, 在治疗过程中未发生移植相关的不良反应, 患者的临床症状得到缓解[9]。越来越多的证据表明, 间充质干细胞是人类自身免疫性疾病细胞治疗的理想细胞选择[10, 11]。
MRL/lpr狼疮小鼠作为SLE的常用动物模型之一, 其症状与人类系统性红斑狼疮相似, MRL/lpr小鼠存在调亡缺陷, Fas基因的突变导致自身反应性淋巴细胞增生, 循环免疫复合物的产生增多, 导致细胞浸润、蛋白尿和进行性肾损害。免疫复合物的沉积最早出现在MRL/lpr小鼠2月龄时, 肾损害为亚急性增生性肾小球肾炎, 轻、中度蛋白尿, 50%死亡率发生于第5个月。本研究以MRL/lpr狼疮鼠作为研究对象, 治疗起始时间选在MRL/lpr小鼠2.5月龄时, 治疗截止时间选在4.5月龄时。
目前的研究表明, 活动性SLE中Th17/Treg细胞免疫平衡被打破, 表现为Th17细胞数目增多, Treg细胞数目减少且缺乏正常免疫活性。通过各种治疗手段使其Th17细胞下降, Treg细胞上调, 可以缓解病情。环磷酰胺是治疗SLE的有效药物之一, 小剂量环磷酰胺治疗SLE疗效确切[12]。
本研究采用经尾静脉移植ADSCs治疗MRL/lpr狼疮鼠, 以环磷酰胺作为治疗对照组。初步探讨移植ADSCs对MRL/lpr狼疮鼠的治疗效果及机制。本研究结果显示, control组狼疮鼠尿蛋白浓度明显高于治疗组, 同时, 脾脏Th17细胞显著高于治疗组, Treg细胞低于治疗组, 提示在MRL/lpr狼疮鼠脾脏细胞中存在Th17/Treg细胞免疫失衡, 且与肾脏受累关系密切。同时观察到移植ADSCs能够减少SLE模型小鼠尿蛋白浓度, 且治疗时间越长, 效果越显著, ADSCs的治疗效果与环磷酰胺的疗效相近。ADSCs能够明显降低狼疮鼠脾脏Th17细胞水平, 提高Treg细胞水平, 使Th17/Treg细胞比值降低, 提示ADSCs能够负向调节狼疮小鼠Th17/Treg细胞平衡, 进而发挥抗炎和免疫调节的作用。机体的免疫系统是一个相互联系、相互影响错综复杂的大网络, 并非几个细胞因子、几条信号途径就可以说明。就现阶段我们的实验结果可见, ADSCs能够通过上调MRL/lpr狼疮鼠脾脏Treg细胞, 减少Th17细胞过度活化来减轻自身免疫反应, 降低MRL/lpr狼疮鼠的肾损害, 从而改善病情。
此外, 间充质干细胞不仅具有免疫调节的能力, 还具有在组织损伤时迁移至损伤部位、修复受损组织的功能。本研究的下一步研究将对治疗前后的MRL/lpr狼疮鼠肾脏进行组织病理检测, 观察ADSCs对MRL/lpr狼疮鼠肾脏的修复情况, 研究移植ADSCs对MRL/lpr狼疮鼠自身抗体、炎性细胞因子以及生存率的影响。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|