目的 探讨 EVI1基因阳性的急性髓系白血病(acute myeloid leukemia, AML)患者的临床生物学特点及其对早期化疗疗效的影响。方法 选择2015年3月至2016年7月在北京大学血液病研究所诊治的361例AML患者病例资料进行回顾性分析,其中33例AML患者 EVI1基因阳性,分析比较其临床特征和生物学特征,并比较中危及高危伴 EVI1+AML患者的临床与生物学特征及诱导缓解率,分析获得完全缓解(complete remission, CR)的影响因素。32例健康供者进行 EVI1/ABL基因水平检测,确定 EVI1表达的异常界值。结果 以 EVI1/ABL基因定量表达≥8.0%作为 EVI1的阳性表达。在361例初发AML中, EVI1+AML患者共33例(9.1%),其中男性16例,女性17例,中位年龄45(18~67)岁,中位随访期为6.6(0.7~13.2)个月。中危核型17例,包括正常核型9例,1例+8;高危核型14例,包括7例-7/7q-,4例t(v;11q23),3例inv(3)/t(3;3),2例未见分裂象。33例患者1个疗程完全缓解率为42.4%,总CR率为60.6%。按《美国国立综合癌症网络(National Comprehensive Cancer Network,NCCN )指南》预后分层,分为中危组16例,高危组17例,无低危组患者。中危组与高危组1个疗程CR率分别为68.8%和17.6%( P=0.005), 总CR率分别为81.3%和41.2%( P=0.032),复发率为7.7%和14.3%。单因素分析,高危染色体核型对1个疗程CR率及总体CR率均有影响( P=0.004、0.029)。高危组患者病死率显著高于中危组(41.2% vs. 6.3%, P=0.039), 且总生存(overall survival, OS)显著低于中危组( P=0.012)。结论 EVI1基因在AML中常伴中、高危核型表达,对于AML患者来说可能不是独立的预后因素,伴-7/7q-、t(v;11q23)及inv(3)/t(3;3)等高危染色体核型的预后差,其1个疗程CR率及总CR率、长期生存率低,病死率高,应尽早行异基因造血干细胞移植。
Objective: To investigate the clinical biological characteristics of EVI1 positive acute myeloid leukemia (AML) and its effect on early chemotherapy.Methods: The clinical and biological cha-racteristics of 33 AML patients with EVI1 positive were retrospectively analyzed in 361 AML patients who were diagnosed and treated in our institute from March 2015 to July 2016, and the clinical and biological features, and rates of the induced remission were compared between the intermediate risk and poor risk with EVI1 positive AML, moreover, the influential factors on complete remission (CR) were analyzed. The expression of EVI1/ABL was tested in 32 healthy donors to confirm the abnormal threshold of EVI1 expression.Results: The definition of EVI1 positive was that the quantitative expression of EVI1/ABL was more than 8.0%. The 33 AML patients with EVI1 positive were found in 361 newly diagnosed AML patients, in which the female and male patients were 17 and 16 respectively, the median age was 45 (18-67) years, with a median follow-up of 6.6 (0.7-13.2) months. Intermediate karyotype was found in 17 patients(including 9 patients with normal karyotypes,1 patient with +8);unfavorable karyotype was found in 14 patients [including 7 patients with -7/7q-,4 patients with t (v;11q23),3 patients with inv(3)/t(3;3), and 2 patients without mitotic figures]. The rate of CR in the first induction chemotherapy was 42.4%, and the rate of total CR was 60.6%. According to the NCCN, 16 intermediate risk patients and poor risk patients were divided, without favorable risk patients. The rate of CR in the first induction chemotherapy were 68.8% and 17.6% ( P=0.005) in the intermediate risk and poor risk respectively, that of total CR were 81.3% and 41.2%( P=0.032), and the rates of relapse were 7.7% and 14.3%.Univariable analysis revealed that unfavorable karyotype could affect the rate of CR in the first reduction chemotherapy and that of total CR ( P=0.004, 0.029). The poor risk patients had higher mortality (41.2% vs. 6.3%, P=0.039) and lower overall survival (OS)( P=0.012).Conclusion: EVI1 may be not an independent prognostic factor for the AML patients considering the appea-rance in the intermediate and poor risk patients. It predicts poor outcome in the EVI1 positive AML patients who have unfavorable karyocytes, such as -7/7q-, t(v;11q23), and inv(3)/t(3;3), and also a low rate of both CR in the first induction chemotherapy and total CR. It also has a low rate of long-term survival and high mortality in the AML patients with EVI1 positive, who may benefit from allogeneic bone marrow transplantation as soon as possible.
急性髓系白血病(actue myeloid leukemia, AML)是一组异质性疾病, 通常以造血干细胞的恶性克隆性增殖及分化成熟受抑为特点。在AML中, 某些患者能检测到原癌基因亲嗜性病毒整合位点 1(ecotropic viral integration site 1, EVI1)的过表达。该基因定位于人类染色体3q26, 编码锌指蛋白, 能特异性地结合基因启动子DNA序列, 发挥转录调节作用。EVI1基因不仅可解除TGF-β 介导的抑制细胞生长的作用, 而且抑制JNK信号通路被激活引发的细胞凋亡[1, 2], 另外, 基因水平的靶向研究结果也表明EVI1对于造血干细胞池的维持及造血干细胞分化是不可或缺的[3]。
在正常人外周血和骨髓中, EVI1基因均有表达, 其表达量分别为 0.5%(外周血)和 1.0%(骨髓)。在EVI1过表达(EVI1+)的AML患者中, 初治时EVI1的中位表达量分别为3.6%(外周血)和23.3%(骨髓)[4]。临床中, EVI1+的AML的发生率为8.0%~21.2%, 易合并inv(3)(q21q26.2)、t(3; 3)(q21; q26.2)、t(v; 11q23)、-7等高危染色体异常, 并且根据《美国国立综合癌症网络(National Comprehensive Cancer Network, NCCN)指南》的预后分型, 多见于中危和高危组患者, 很少见于低危组患者(1% vs. 99%); 对于完全缓解(complete remission CR)率、无事件生存(event free survival, EFS)率, 尤其是中危组的AML患者, EVI1+都是独立的不良因素[5, 6, 7]。EVI1+中危AML患者与EVI1+高危AML患者是否具有相似的临床特点, 两者间疗效及总生存(overall survival, OS)时间是否有所区别, 目前尚未见报道, 因此, 本研究对33例EVI1+AML患者按NCCN指南进行了低、中、高危分组, 并对其临床特征、生物学特征及诱导缓解率、OS做了比较分析。
选择2015年3月至 2016年7月北京大学血液病研究所住院治疗的361例AML患者进行回顾性队列分析, 其中33例AML患者EVI1+, 随访日期截止至2016年9月30日。所有患者均签署知情同意书, 常规经骨髓穿刺, 行骨髓细胞形态、免疫分型、基因、染色体检查, 根据骨髓细胞形态学、免疫学、细胞遗传学、分子生物学(morphology immunology cytogenetics molecular biology, MICM)诊断分型标准确诊分型, 患者均无严重心、肺、肝、肾功能不全和重症感染, 美国东部肿瘤协作组(Easterm Cooperative Oncology Group, ECOG)评分≤ 2分。
以2015年4月至2016年7月于北京大学血液病研究所行造血干细胞采集的32例健康造血干细胞移植供者为正常参考人群, 测定其骨髓细胞EVI1基因的表达水平。
骨髓血经肝素抗凝, 应用四色荧光直接免疫标记法流式细胞术检测, 以CD45/SSC(侧向散射)射门, 检测北京大学血液病研究所初治的急性白血病常规标记抗原[8]。
骨髓血经肝素抗凝, 经过24 h培养制备染色体, 应用G显带技术, 参照《人类细胞遗传学国际命名体制(ISCN2009)》进行核型分析。
骨髓血经EDTA抗凝, 用RNA通过实时定量PCR(RQ-PCR)方法检测EVI1、AML1-ETO、PML-RARa、CBFβ -MYH11、MLL等基因, 另外用DNA通过定量PCR检测NPM1(A、B和D型)及FLT3-ITD和FLT3-TKD基因突变, 以ABL基因为内参, 靶基因表达水平=靶基因拷贝数/ABL拷贝数× 100%[9]。通过PrimerExpress Software设计EVI1基因(包括1a、1b、1c和3L 4种亚型)的引物和探针序列。上游引物为5'-CCCATGTGCCAGAGGAACTT-3'(14号外显子), 下游引物为5'-CAGTGACAGCATCATAGCATATGC-3'(15号外显子), 探针为5'-FAM-CAGCCGTTACACAGAAAGTCCAAATCGC-TAMRA-3'(14号外显子)。
AML患者采用IA方案(去甲氧柔红霉素 8~10 mg/m2× 3 d, 阿糖胞苷 100 mg/m2× 7 d)、MA方案(米托蒽醌 6 mg/m2× 3 d, 阿糖胞苷 100 mg/m2× 7 d)、DA方案(柔红霉素60 mg/m2× 3 d, 阿糖胞苷 100 mg/m2× 7 d)或CAG方案[阿克拉霉素 20 mg× 4 d, 阿糖胞苷 10 mg/m2(q12h)× (7~14 d), 重组人粒细胞刺激因子 300 μ g × (7~14 d), 根据外周血白细胞情况加或不加地西他滨(20 mg/m2× 5 d)进行诱导治疗, 巩固强化治疗采用中剂量阿糖胞苷[2 g/m2(q12 h)× 3 d], 获得CR后, 化疗 2~3个疗程, 根据危险度分层选择是否继续化疗或行异基因造血干细胞移植, CR标准参见既往报道[10]。
采用SPSS 19.0软件进行统计分析, 率的比较采用精确概率检验; 计量资料以均数± 标准差表示, 组间比较采用独立样本t检验; 生存分析采用Kaplan-Meier法和log-rank检验, P< 0.05认为差异有统计学意义。
健康供者EVI1基因表达量中位数为 2.9%(0.6%~7.1%)。在361例AML中, 有33例患者EVI1基因过表达, 发生率为9.1%, 其中北京大学人民医院初治患者21例。33例患者中, 男性16例(48.5%), 女性17例(51.5%), 中位年龄45(18~67)岁, 中位随访时间6.6(0.7-13.2)个月。伴MDS(myelodysplastic syndrome)相关改变或前期肿瘤治疗病史者6例(18.2%)。正常核型和其他未定义中危核型占48.5%(16/33), 高危占42.4%(14/33), 其中-7/7q-高达21.2%(7/33), t(v; 11q23)占12.1%(4/33), inv(3)/t(3; 3)占9.1%(3/33), 1例+8, 2例未见核分裂象。21例北京大学人民医院初治患者EVI1基因表达量中位数为86.0%(14.0%~346.7%)。在基因异常病例中, MLL基因异常8例(24.2%), FLT3-ITD突变4例(12.1%), FLT3-TKD突变1例(3.0%), CEBPα 单突变3例(9.1%), NPM1突变1例(3.0%)。按NCCN指南预后分层, 低危组0例(0%), 中危组16例(48.5%), 高危组17例(51.5%, 表1)。
| 表1 33例EVI1+AML患者一般临床资料及特点 Table 1 The clinical data and characteristics of 33 AML patients with EVI1 positive |
33例EVI1+患者均为髓系表达, 免疫分型多数表达髓系干组细胞标记[CD117(84.8%)、HLA-DR(75.8%)、CD33(72.7%)、CD34(60.6%)、CD38(51.5%)、CD123(42.4%)、CD13(69.7%)], 另外有部分病例伴CD64、CD56、CD7、CD36、CD11c、CD11b、CXCR4表达, 仅有3例表达cMPO。
中危组患者正常核型及其他未定义中危核型分别占37.5%和43.8%, 高危组患者, 伴高危核型异常占82.4%, 主要为-7/7q-, 占41.2%, 其次为t(v; 11q23)、inv(3)/t(3; 3), 分别占23.5%和17.6%。两组患者均有合并MLL异常者, 但均无合并AML1-ETO融合基因及单纯CEBPα 双突变、CBFβ -MYH11等预后良好基因; 在2例染色体无分裂象的病例中, 1例无特殊基因异常, 1例为NPM1突变伴FLT3-TKD突变, 均为中危组患者(表2)。
| 表2 33例EVI1+AML患者细胞遗传学及分子生物学分布 Table 2 The cytogenetics and molecular biology distribution of 33 AML patients with EVI1 positive |
33例EVI1+患者中, 14例经1个疗程诱导获得CR, CR率为42.4%(14/33), 累积2个疗程CR率为 54.5%(18/33), 累积3个疗程CR率为60.6%(20/33), 总CR率为60.6%(20/33)。EVI1+患者高危组1个疗程CR率、总CR率均低于中危组(P=0.005、0.032), 高危组患者病死率明显高于中危组(P=0.039)。中危组13例CR患者中1人复发, 高危组7例CR患者中1例复发, 两组CR患者复发率差异无统计学意义(17.7% vs. 14.3%, P=1.000, 表3)。
| 表3 两组患者疗效比较 Table 3 The treatment effect comparison between two groups of patients |
单因素分析, 染色体核型(中危核型vs.高危核型)对1个疗程CR率(72.2% vs. 14.3%, P=0.004)及总CR率(83.3% vs. 35.7%, P=0.029)均有影响; 初诊时EVI1基因表达水平对1个疗程CR率及总CR率均无影响。
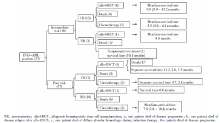
33例EVI1患者治疗及转归情况见图1, 高危组患者OS低于中危组患者(P=0.012, 图2)。
EVI1基因在AML患者中过表达的发生率为8.0%~21.2%, 发病时中位年龄为37~51岁, 发病时白细胞、血小板中位数、男女发病率、原始细胞数与EVI1阴性AML患者比较, 差异无统计学意义[5, 6, 7]。不同实验室由于不同因素影响, 骨髓中EVI1基因正常表达的阈值也各不相同(1%~12%)[4, 7], 本研究根据测得的正常人骨髓中EVI1基因表达的结果, 将EVI1基因过表达的阈值设定为8%。
EVI1基因位于人类染色体3q26, 其编码的锌指蛋白参与调控造血干细胞的自我更新及多种再生活性[11]。在伴有inv(3)(q21q26.2) 或t(3; 3)(q21; q26.2)异常的AML患者常伴有EVI1基因的过表达[12, 13], 然而在EVI1+AML中, 也常合并除涉及3q26以外的其他染色体异常。相关研究表明[6, 7, 14, 15], EVI1+AML患者, 其染色体常伴有-7/7q-或t(11q23)的异常(P< 0.05); 在伴有t(v; 11q23)异常的EVI1+AML患者中, t(6; 11)最常见(83.9%), 其次为t(9; 11)(83.9%); 此外EVI1阳性常见于中高危组; EVI1+AML患者中很少伴有t(8; 21)、inv(16)、t(15; 17) 、NPM1突变、CEBPα 双突变(P< 0.05)等预后良好基因的表达。本研究EVI1+AML无低危核型分布, 伴有inv3(q21q26)、t(11q23)、-7/7q-的表达, 尤其在高危患者中-7/7q-比例高达 41.2%, 且在细胞遗传学中, 伴有24.4%的患者伴有MLL基因异常, 基本符合国外报道[5]。
研究发现[5, 16], EVI1基因可以作为AML预后不良的分子生物学标记, EVI1+AML患者CR率小于EVI1-AML患者(53% vs. 77%, P< 0.01), 且是中、高危组独立的预后不良因素。国内有相关报道发现EVI1高表达AML患者第1个疗程CR率低于EVI1低表达患者[17]。本研究总CR率为60.6%, 与文献报道基本相符[6]。本研究发现EVI1+AML中危组患者CR率、OS高于高危组, 且病死率低。EVI1基因对于AML患者, 存在于中危及高危人群中, 单独EVI1基因表达可能不是AML患者独立预后因素, 当其伴随高危细胞遗传学或分子生物学表达时, 会使该类患者预后更差。
 | 图2 中、高危患者总生存比较Figure 2 The survival comparison between intermediate patients and unfavorable patients |
目前国外学者认为[6], 对于EVI1+AML患者, 在首次缓解后行异基因造血干细胞移植治疗可以改善5年无复发生存。虽然本研究随访时间短, 且病例数较少, 但是仍能从图1中看出, 到观察截止时(中位观察期6.6个月), 接受allo-HSCT的中危患者9/9生存, 高危4/5生存。接受化疗的中危组患者6/7生存, 高危组6/12生存。2例NR患者移植后至观察截止日生存6个月和8个月。因此可以认为对于EVI1+AML患者, 异基因造血干细胞移植也是提高疗效的方法。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|