目的: 探讨雷公藤甲素(triptolide ,TP)对TM4小鼠睾丸支持细胞氧化应激、凋亡的影响及相关分子信号调控机制。方法: 不同浓度雷公藤甲素对TM4细胞染毒24 h;细胞增殖实验检测雷公藤甲素对TM4细胞增殖的抑制作用;通过2',7'-二氯荧光黄双乙酸盐(6-carboxy-2',7'-dichlorofluorescein diacetate,DCFH-DA)探针检测TM4细胞内活性氧水平的变化;Annexin V/PI双染检测雷公藤甲素诱导TM4细胞凋亡变化; Western blot免疫印迹法分别检测凋亡标志蛋白cleaved-PARP和PI3K/Akt通路相关蛋白表达情况。结果: 细胞增殖实验提示雷公藤甲素对TM4细胞有显著抑制作用,呈浓度梯度上升[10 nmol/L:(73.77±20.95)%, 100 nmol/L:(51.60±10.43)%, 500 nmol/L:(44.34±5.78)%];雷公藤甲素处理使细胞内活性氧水平上调( P<0.01),呈浓度依赖性;给药组TM4细胞早期凋亡和晚期凋亡比例均上调[对照组:(3.84±1.50)%, 100 nmol/L:(13.04±2.03)%, 200 nmol/L:(16.24±1.34)%, 400 nmol/L:(18.76±3.45)%],各给药组cleaved-PARP表达上调( P<0.01);给药组TM4细胞中Akt、p70S6K磷酸化水平上调( P<0.01),mTOR磷酸化水平未见显著变化( P>0.05)。结论: 在体外培养条件下,雷公藤甲素可抑制TM4睾丸支持细胞增殖,并诱导TM4细胞凋亡增加,呈浓度依赖性;雷公藤甲素同时可引起TM4细胞中氧化应激水平上调,这可能是其细胞毒性机制之一;雷公藤甲素可导致Akt和p70S6K的激活,磷酸化水平升高,提示PI3K/Akt信号通路可能参与雷公藤甲素诱导的TM4细胞氧化应激反应,激活PI3K/Akt信号通路可能是其分子调控机制之一。
*These authors contributed the work equally
Objective: To investigate the effect of triptolide (TP) on oxidative stress and apoptosis in TM4 sertoli cells and related molecular mechanism.Methods: TM4 cells were incubated with different concentrations of triptolide for 24 h, then collected for further experiments. Cell proliferation analysis was used to measure the inhibitive effect of triptolide on proliferation of TM4 cells; DCFH-DA (6-carboxy-2',7'-dichlorofluorescein diacetate) probe was used to stain the TM4 cells, the level change of intracellular ROS was discovered through flow cytometry; the TM4 cells were stained by Annexin V-FITC/PI to detect whether triptolide induced apoptosis in the TM4 cells; Protein was extracted from the TM4 cells in control and triptolide group. Western blot was performed to determine the expression of apoptosis marker protein cleaved-PARP and PI3K/Akt signaling pathway-related proteins [p-Akt (Ser473), Akt, p-mTOR (Ser2448), mTOR, p-p70S6K (Thr389), p70S6K].Results: Cell proliferation analysis revealed that triptolide reduced the TM4 cells viability significantly compared with control group in a dosage-dependent manner [10 nmol/L: (73.77±20.95)%, 100 nmol/L: (51.60±10.43)%, 500 nmol/L: (44.34±5.78)%]; The level of intracellular ROS in the TM4 cells was significantly induced in a dosage-depen-dent manner ( P<0.01); triptolide remarkably induced early-stage and late-stage apoptosis in the TM4 cells [control: (3.84±1.50)%, 100 nmol/L: (13.04±2.03)%, 200 nmol/L: (16.24±1.34)%, 400 nmol/L: (18.76±3.45)%]; The expression of cleaved-PARP was significantly upregulated in the TM4 cells after incubation with triptolide ( P<0.01); The expression levels of p-Akt/Akt and p-p70S6K/p70s6k were significantly increased compared with control group ( P<0.01). No significant change was observed among the expression levels of p-mTOR/mTOR ( P>0.05).Conclusion: In vitro studies showed that triptolide could effectively suppress the proliferation and induce apoptosis of TM4 sertoli cells. The oxidative stress was upregulated after incubation with triptolide, which may be one of the mechanisms of cytotoxicity in TM4 cells. Treatment of triptolide led to activation of Akt and p70S6K, indicating that the PI3K/Akt signaling pathway may be involved in response to oxidative stress in TM4 cells. The activation of PI3K/Akt signaling pathway was one of the molecular mechanisms involved in triptolide-mediated oxidative stress in TM4 cells. Our study provides insight into alleviating reproductive toxicity of triptolide in clinical and developing male contraceptive.
雷公藤(Tripterygium wilfordii Hook.f.)是卫矛科雷公藤属的一种植物, 其根部提取物雷公藤多苷在临床上应用广泛, 多用于自身免疫性疾病的治疗。在临床应用的过程中, 长期服用雷公藤多苷会对男性生殖内分泌系统造成损害。随着传统中医药的发展, 从雷公藤的根部提取出一种环氧二萜内酯类化合物— — 雷公藤甲素(triptolide , TP), 是雷公藤多苷的主要活性成分之一[1]。既往众多研究表明, 雷公藤甲素具有显著的抗炎、抗肿瘤以及抗生育等生物活性[2, 3, 4], 另一方面, 雷公藤甲素的肝毒性、肾毒性, 特别是生殖毒性限制了其临床应用。目前有关雷公藤抗生育作用具体分子机制的研究相对较少见, 特别是对支持细胞的影响尚未完全明确。
生精小管由处于不同阶段的生精细胞同心层组成, 睾丸支持细胞穿插其中, 支持细胞胞质围绕生精细胞延伸, 提供物理支持并形成精子发生的稳定环境[5], 同时为精原细胞的存活和生长提供养分[6]。因此, 深入探究影响睾丸支持细胞数量和功能的因素, 对于更好地了解精子发生过程十分重要。睾丸支持细胞的特点是对有毒物质和药物非常敏感, 可以突显化合物对于精子发生的潜在毒性[7]。本研究以TM4小鼠睾丸支持细胞株作为研究对象, 探讨雷公藤甲素对TM4细胞氧化应激和凋亡水平的影响以及PI3K/Akt信号通路的变化, 旨在从细胞水平探讨雷公藤抗生育作用涉及的潜在分子机制, 对减轻其生殖毒性、扩大临床应用范围提供新的思路。
DMEM/F12培养基、胰蛋白酶、胎牛血清为美国Gibco公司产品; 雷公藤甲素和DCFH-DA为美国Sigma公司产品; 兔抗Akt、p-Akt(Ser473)、mTOR、p-mTOR(Ser2448)、p70S6K、p-p70S6K(Thr389)、cleaved-PARP抗体和辣根过氧化物酶 (horseradish peroxidase, HRP)标记的羊抗兔、抗鼠IgG为美国CST公司产品。小鼠抗β -actin抗体为碧云天公司产品。
小鼠睾丸支持细胞株TM4购于美国ATCC公司, 常规培养于含10%(体积分数)胎牛血清的DMEM/F12培养基中。培养环境为37 ℃ 95%(体积分数)空气、5%(体积分数)二氧化碳。每3天根据细胞状态和密度进行换液或传代, 取对数生长期细胞进行实验。
将TM4细胞按密度为5× 103 个/mL接种于96孔板, 待过夜细胞贴壁后, 将各孔更换为包含不同浓度雷公藤甲素的培养基。每组药物浓度设6个复孔, 药物终浓度为10、100、500 μ mol/L, 另设阴性对照加入等量二甲基亚砜(dimethyl sulfoxide, DMSO)。加药处理24 h后, 弃去培养基, 加入含有四唑化合物[3-(4, 5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt, MTS]试剂(美国Promega公司)的培养基, 37 ℃孵育2 h。使用酶标仪(美国Thermo Fisher公司)分别于490 nm和690 nm处测定每孔的光密度值。细胞活性用百分比表示。
细胞凋亡使用Annexin V-FITC/PI双染细胞凋亡试剂盒进行检测。将TM4细胞接种于6孔板中(细胞密度为5× 105个/ mL), 待细胞生长48 h后, 给药组使用100~400 nmol/L雷公藤甲素处理 24 h, 另设阴性对照组加入等量DMSO。用不含EDTA的胰酶消化, 2 000 r/min离心5 min, 冰PBS洗涤两次。收集5× 105 个细胞并重悬于500 μ L染色缓冲液, 加入5 μ L PI和5 μ L Annexin V-FITC后混匀, 室温避光孵育15 min。使用FACScan(美国BD公司)流式细胞仪进行检测, 每组样本共收集 20 000个细胞。
利用2', 7'-二氯荧光黄双乙酸盐(6-carboxy-2', 7'-dichlorofluorescein diacetate, DCFH-DA)作为分子探针进行细胞内活性氧的检测, 将TM4细胞接种于6孔板中, 经过雷公藤甲素处理24 h后, 以体积比1 :1 000用无血清培养基稀释DCFH-DA, 使其终浓度为10 μ mol/L。去除培养基, 加入稀释好的DCFH-DA, 37 ℃孵育20 min。用无血清培养基洗涤细胞3次, 收集细胞后用流式细胞仪于488 nm激发波长、530 nm发射波长进行检测。
收集TM4细胞, 冰PBS洗涤3遍, 加入裂解液(含蛋白酶抑制剂和磷酸酶抑制剂), 冰上裂解30 min。悬液用12 000 × g离心10 min, 取上清液。使用BCA法进行蛋白浓度测定, 用裂解液将各组蛋白调整至相同浓度, 加入5× 上样缓冲液, 100 ℃煮沸10 min使蛋白变性。变性后的蛋白(每个上样孔20 μ g)经SDS-PAGE电泳(6%~10%分离胶)分离后, 湿转2 h至PVDF膜上。TBST配置的5%(体积分数)牛血清白蛋白室温封闭1 h。一抗经TBST稀释, 体积比为1 :1 000, 4 ℃孵育过夜。TBST漂洗3次, 每次10 min。羊抗兔、抗鼠IgG二抗室温孵育1 h, TBST漂洗3次, 每次10 min。配置ECL化学发光试剂, 混匀后敷在PVDF膜上。使用SYNGENE曝光系统拍照。
使用SPSS 19.0软件, 采用单因素方差分析和t检验等方法分析数据, 计量资料以均数± 标准差表示。实验至少重复3次, P< 0.05认为差异有统计学意义。
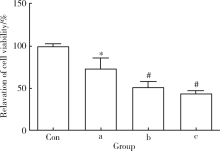
通过MTS细胞增殖实验检测雷公藤甲素对TM4细胞活力的影响, 分别采用10、100、500 nmol/L浓度的雷公藤甲素处理TM4细胞24 h, 发现10 nmol/L浓度的雷公藤甲素对TM4细胞的增殖有显著的抑制(图1), 随着药物浓度的提高, 抑制作用呈浓度梯度上升[10 nmol/L:(73.77± 20.95)%, 100 nmol/L:(51.60± 10.43)%, 500 nmol/L:(44.34± 5.78)%]。各组药物浓度下, TM4细胞的活力相较对照组差异具有统计学意义(P< 0.05或P< 0.01)。
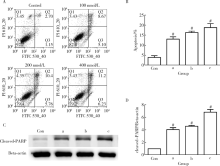
为了明确雷公藤甲素是否对TM4细胞氧化应激水平有影响, 实验选取了DCFH-DA作为荧光探针, 用于检测细胞内活性氧水平。采用100~400 nmol/L雷公藤甲素处理TM4细胞24 h后, 给药组细胞内活性氧水平显著上调, 呈浓度依赖性, 各给药组相较于对照组差异具有统计学意义(P< 0.01, 图2)。
对雷公藤甲素处理过的TM4细胞进行Annexin V-FITC/PI双染色, 随后通过流式细胞仪检测细胞凋亡水平, 图3A显示相较于对照组, 给药组TM4细胞早期凋亡和晚期凋亡比例均上调, 随浓度而增加, 图3B显示各给药组凋亡细胞百分数与对照组相比显著增加[对照组:(3.84± 1.50)%, 100 nmol/L:(13.04± 2.03)%, 200 nmol/L:(16.24± 1.34)%, 400 nmol/L:(18.76± 3.45)%, P< 0.01]。通过Western blot检测细胞凋亡标志物cleaved-PARP的表达, 图3C、D显示各给药组cleaved-PARP表达显著上调(P< 0.01)。以上两个实验证实了雷公藤甲素可引起TM4细胞凋亡增加。
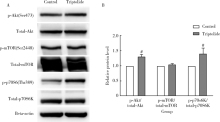
TM4细胞经过100 nmol/L雷公藤甲素处理24 h后, 提取蛋白并通过Western blot检测p-Akt(Ser473)、Akt、p-mTOR(Ser2448)、mTOR、p-p70S6K(Thr389)、p70S6K的表达(图4), Akt和p70S6K在给药组被激活, 磷酸化水平显著上调, 磷酸化mTOR水平无明显变化。
睾丸支持细胞定位于生精小管的基底膜附近, 相邻的支持细胞主要通过紧密连接形成血睾屏障。血睾屏障可形成和维持正常的生精微环境, 防止有毒物质对精子发生过程产生干扰。同时睾丸支持细胞为发育中的生精细胞提供支持和营养, 从生精小管截面图片可以清楚地显示支持细胞和生精细胞间紧密的联系, 这种紧密联系使得生精细胞可以从支持细胞中获得发育所必须的营养物质和其他分子[8]。睾丸支持细胞的结构和功能对于精原细胞的增殖和精子发生十分关键, 支持细胞任何的异常变化都有可能导致精子发生障碍[6, 9]。
基于社会、经济、实施难度以及伦理道德等方面考量, 在过去的几十年中, 毒理学的研究重点已经逐渐从基于动物的实验转变为体外研究。体外模型主要包括原代细胞和永生化细胞系两种类型的细胞, 相对于原代培养的细胞, 细胞系更容易获得并且不需要从动物身上提取。在诸多针对毒性物质影响男性生育力的研究中, TM4细胞系是应用最为广泛的。TM4细胞持续表达睾丸支持细胞的一些特性, 例如分泌转铁蛋白和纤溶酶原激活物, 同时表达雄激素和雌激素受体, 对卵泡刺激素也有反应[10]。因此, 本研究选取TM4小鼠睾丸支持细胞系用于评价雷公藤甲素的生殖毒性。
本实验结果中, 细胞增殖实验显示雷公藤甲素浓度为10 nmol/L时就会对细胞生长产生抑制作用, 当浓度提高至100 nmol/L, 细胞存活率低于50%, 证实了雷公藤甲素可抑制TM4细胞的增殖。既往研究发现[11], 雷公藤甲素对睾丸支持细胞的毒性作用, 实验使用的浓度为125~500 nmol/L。本研究选取了100~400 nmol/L浓度进行后续实验, 在TM4细胞经过24 h处理后, 细胞内活性氧水平显著上调, 进一步通过流式细胞学和Western blot证实雷公藤甲素可诱导TM4细胞的凋亡, 同时, 发现在PI3K/Akt信号通路中, Akt和p70S6K磷酸化水平显著上调, Akt和p70S6K总蛋白表达没有变化, 磷酸化的mTOR水平没有显著变化。既往有研究从内分泌以及Nrf2抗氧化通路等角度探讨雷公藤甲素对睾丸支持细胞的毒性作用[11, 12], 本研究与其研究结果相一致, 均证实了雷公藤甲素可抑制支持细胞活力, 并诱导凋亡的增加, 同时, 本研究还发现PI3K/Akt信号通路在这个过程中被激活, 为日后深入探讨雷公藤甲素生殖毒性提供了线索。
氧化应激的发生, 通常是因为活性氧的产生与抗氧化系统清除不平衡所致, 既往研究表明[13, 14, 15, 16], 在不同器官中雷公藤甲素可通过增加氧化应激水平产生毒性作用。适宜水平的活性氧与细胞分化以及精子发生的生理过程密切相关[17], 但活性氧水平的异常升高会导致生精细胞的凋亡, 损害正常的生精过程[18, 19, 20]。本研究雷公藤甲素诱导了TM4细胞中活性氧的产生, 进一步导致TM4细胞凋亡, 流式细胞学结果提示TM4细胞经过雷公藤甲素处理后, 早期凋亡和晚期凋亡比例显著上调。同时, PARP作为一个经典的凋亡分子标志物[21], 本研究通过Western blot检测到在药物组其剪切体的表达显著增加, 认为在睾丸支持细胞中, 雷公藤甲素诱导的活性氧生成, 进一步激活了细胞凋亡通路, 是其发挥生殖毒性的重要作用机制之一。
PI3K/Akt信号通路与细胞的增殖和存活密切相关, 该通路的激活可通过一系列磷酸化作用发挥抗凋亡的功能[22, 23]。同时, 大量研究证实该通路在精子发生过程中发挥重要作用, 参与精原干细胞的自我更新[24, 25]。在支持细胞分泌的物质中, 乳酸是为精母细胞和精子细胞提供能源的首要物质。Riera等[26]的研究表明, 在降低支持细胞培养环境中葡萄糖水平后, 乳酸的产量只有轻微的减少, 同时PI3K/Akt通路显著激活, 提示在支持细胞中该通路的激活与细胞适应异常生存条件有关。还有学者发现细胞内氧化应激水平提高后[27, 28], 可引起PI3K/Akt信号通路的代偿性激活, 进而调节HO-1的表达和Nrf2的转录激活, 起到抗氧化应激的作用。本研究也发现TM4细胞活性氧水平的上调, 导致了Akt和p70S6K的激活, 磷酸化水平升高, 提示PI3K/Akt信号通路参与到雷公藤甲素诱导的TM4氧化应激反应中, 发挥了抗氧化功能。
综上所述, 本研究发现雷公藤甲素可以抑制TM4小鼠睾丸支持细胞的增殖, 并使细胞内活性氧水平异常升高, 导致细胞凋亡通路的激活。雷公藤甲素引起的PI3K/Akt信号通路激活, 可能与TM4细胞内氧化应激水平的变化相关, 这可能是雷公藤发挥抗生育作用涉及的新的分子机制。本研究为临床上使用雷公藤治疗的同时降低其生殖毒性, 以及利用此分子机制开发男性避孕药提供了新的思路。
(本文编辑:王 蕾)
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|