肾血管平滑肌脂肪瘤(angiomyolipoma, AML)是泌尿系统常见的良性肿瘤,主要由脂肪组织、血管及肌肉组织构成。肾AML可同时合并结节性硬化症,但多数为散发,好发于中年女性,无明显临床症状,可由影像学检查检出。典型AML在CT平扫上具有特征性的脂肪密度,易与其他肾肿瘤相鉴别,但少部分AML含有极少量或不含有脂肪成分,平扫CT难以发现负值成分,称为乏脂型血管平滑肌瘤,其确诊依赖组织病理检查结果。另外,部分AML具有恶性表象,可累及肾周、肾窦、淋巴结、其他内脏器官及下腔静脉,进一步增加了鉴别诊断的难度。目前国内尚未见乏脂型肾血管平滑肌脂肪瘤伴发淋巴结肿大的相关报道。本文报道1例27岁女性患者,无结节性硬化症家族史,初因CT发现左肾旁团块状高密度影及颈部、腋窝、腹股沟、腹腔及腹膜后多发淋巴结肿大就诊于血液科,考虑淋巴瘤可能,为明确诊断而转入泌尿外科行腹腔镜下左肾周肿物、腹膜后淋巴结切除术和活体组织检查,术后组织病理结果提示AML。患者术后并发持续性淋巴瘘,腹膜后引流乳糜液100~200 mL/d,持续12周,予禁食、补液等保守治疗措施后成功闭瘘。本文回顾了国内外的相关病例及文献,并对伴多发淋巴结肿大的肾AML诊断、治疗和术后顽固性淋巴瘘的处理进行总结和探讨。
SUMMARY Renal angiomyolipoma (AML) is a common benign tumor in the urinary system, mainly composed of adipose tissue, blood vessels and muscle tissue. Renal AML is sporadic in most of patients, while a few are associated with tuberous sclerosis. Classical renal AML occurs predominantly in middle⁃aged females. Most cases are found incidentally during imaging examinations. The fat content makes AML have unique imaging characteristics and is easy to be identified with other renal tumors. However, the amount of fat varies in each tumor. AML that contains only microscopically detectable fat and whose amount of intratumoral fat may be too small to be identified on unenhanced computed tomography (CT) images is termed minimal fat or fat⁃poor renal AML, which appears as a high density shadow in the renal parenchyma on unenhanced CT images. Thus, it can be difficult to distinguish it from renal cell carcinoma (RCC) on imaging. Since the imaging findings are atypical, the diagnosis depends on pathological results. In addition, a few of AML can mimic malignant neoplasms. Recent studies suggested that AML might involve to peri⁃renal or renal sinus fat, regional lymphatics and other visceral organs, as well as inferior vena cava, which further makes the diagnosis more difficult. However, there is currently no reports about involvement of regional limphatics in minimal fat renal AML. In this article, we report a 27⁃year⁃old female patient without family history of tuberous sclerosis, who came to visit the hematologist because a high density shadow near the left kidney was found during CT scan which was accompanied by neck, armpits, groin, abdominal cavity and retroperitoneal lymph nodes enlargement. She was suspected of lymphoma in the beginning and transferred to Department of Urology to perform laparoscopic left renal mass and retroperitoneal lymph node excision and pathological examination for a definitive diagnosis. Finally, pathologic results revealed AML. Postoperative continuous lymphatic fistula developed and the retroperitoneal drainage of chylous fluid was 100-200 mL per day, lasting for 12 weeks. The fistula was successfully closed after conservative treatment including fasting and rehydration. This article summaries and discusses the diagnosis and treatment of renal AML with lymph nodes enlargement and the management of postoperative refractory lymphatic fistula by reviewing the related cases and literature.
肾血管平滑肌脂肪瘤(angiomyolipoma, AML)又称肾错构瘤, 是泌尿系统常见的良性肿瘤, 主要由脂肪组织、血管及肌肉组织构成。典型错构瘤内部富含脂肪组织, B超、CT及MRI有特征性影像学表现, 可提示诊断。然而, 约4.5%的错构瘤内部脂肪含量较低[1], 平扫CT下难以发现负值成分, 仅在组织病理检查时可见少量脂肪成分, 被称为乏脂型AML, 难以与肾恶性肿瘤相鉴别[2]。AML虽作为一种良性肿瘤, 但可累及肾周、肾窦、淋巴结、其他内脏器官及下腔静脉等部位[3, 4], 具有恶性表象, 难以与晚期恶性肾肿瘤相鉴别。基于上述, 同时具有淋巴结肿大和CT下难以发现脂肪成分这两种特点的肾AML极易误诊, 目前国内尚未见相关报道。本文报道1例伴发腋窝、腹腔、腹膜后等淋巴结肿大的乏脂型肾AML, 并结合患者术后顽固性淋巴瘘的处理来探讨这一疾病的临床特点及诊断处理难点, 为实际临床工作总结经验并提供思路。
患者女性, 27岁, 主因“ 发现左肾旁占位合并全身多发淋巴结肿大1月余” 于2017年9月25日入院。患者2017年8月体格检查腹部彩超显示左肾旁团块状高密度影, 大小约4 cm× 6 cm, 内可见血流信号, 无腰痛、血尿、尿频、尿急等不适。
2017年9月3日患者就诊行泌尿系增强CT示左肾脂肪囊内可见团块状高密度影, 大小约3.4 cm× 1.7 cm× 6.4 cm, 密度均匀, 平扫CT值约44 HU, 增强扫描可见明显较均匀强化, CT值约70 HU。肿块边界尚清晰, 与左肾关系密切, 局部左肾受压(图1)。腹膜后可见多发肿大淋巴结, 较大者约1.8 cm× 1.3 cm, 平扫CT值约28 HU, 增强扫描可见强化, 部分强化不均匀, CT值45~80 HU(图2)。彩超检查示颈部、腋窝、腹股沟、腹腔及腹膜后多发淋巴结肿大(图3)。
患者就诊于血液科, 考虑淋巴系统疾病可能, 因颈部、腋窝、腹股沟等浅表部位淋巴结彩超表现不典型, 不除外生理性改变, 故建议行左肾周肿物及腹膜后淋巴结活体组织检查以明确诊断, 于2017年9月25日转入北京大学人民医院泌尿外科进一步诊治。患者无家族性结节性硬化症病史, 无肾肿瘤家族史。
入院体格检查:生命体征平稳, 面部未见皮脂腺瘤, 肢端未见甲床下纤维瘤, 全身未见皮肤色素脱失斑, 心、肺、腹无异常, 双侧肾区无膨隆、无叩痛, 双肾下极未触及, 双侧输尿管走行区无压痛, 膀胱区叩诊鼓音。
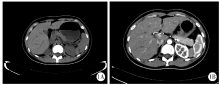 | 图1 平扫(A)及增强(B)CT示左肾旁肿物Figure 1 The mass beside the left kidney was showed on plain (A) and contrast enhanced (B) computed tomography scan |
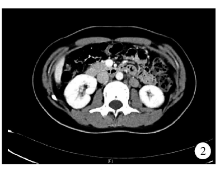 | 图2 增强CT示腹膜后及腹腔多发淋巴结肿大Figure 2 Multiple abdominal and retroperitoneal lymph nodes were identified on enhanced computed tomography imaging |
诊疗经过:入院后完善各项化验检查未见明确手术禁忌证, 2017年9月27日于全身麻醉下以腹腔镜经腹膜后腔行左肾周肿物、腹膜后淋巴结切除活检术。手术过程中在肾背侧肾动脉下方见一枚直径约1.8 cm淋巴结, 切除后利用Hemolok夹闭淋巴结根部以避免淋巴瘘。术中打开肾脂肪囊后可见肾中部偏腹侧直径约4 cm肿物突出于肾表面, 基底与肾关系致密, 将肾周肿物自肾表面剥除, 肾脏创面无明显出血, 未行特殊处理。肿物组织糟脆, 放置腹膜后引流管后缝合切口。患者术中生命体征平稳, 出血约10 mL。术后左肾周肿物及腹膜后淋巴结组织病理检查结果:肿瘤细胞呈梭形, 细胞排列呈束状, 细胞中度异型, 部分区域可见分化成熟的脂肪及厚壁血管。免疫组织化学染色结果:S-100(-)、Sox-10(-)、desmin(+)、CD34(血管+)、CgA(-)、Syn(-)、β -catenin(部分+)、myogenin(+)、SMA(+)、HMB45(灶+)、MelanA(-), 符合AML(图4)。
 | 图3 超声示腋窝(A)、腹膜后腹主动脉旁(B)淋巴结肿大Figure 3 Axillary (A) and retroperitoneal (B) lymph nodes were identified on ultrasound |
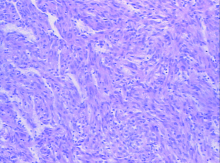 | 图4 组织病理检查示血管平滑肌脂肪瘤, 包含平滑肌、血管及较少量脂肪成分(HE × 100)Figure 4 Minimal fat renal angiomyolipomas consisted of smooth muscle cells, blood vessels, with only small amounts of fat (HE × 100) |
术后患者腹膜后持续引流淡红色血性液体, 最多达400 mL/d, 术后第7天转乳糜状液体, 100 mL/d, 考虑并发术后淋巴瘘, 予清淡饮食、引流管负压吸引治疗, 引流量逐渐减少, 最少约60 mL/d, 伤口愈合良好, 于2017年10月9日出院。出院后随访, 患者腹膜后持续引流乳糜状液体, 100~200 mL/d, 进食“ 黄油” 等食物后引流量明显增多, 进食清淡食物后引流量则较少, 引流管通畅。
术后12周患者因持续性淋巴瘘再次入院。入院体格检查:生命体征平稳, 皮肤、黏膜温度、湿度及弹性可, 心、肺无异常, 腹平软, 左下腹腋前线引流管通畅, 引流液色乳白, 量约100 mL。全腹无紧张、压痛及反跳痛, 双侧肾区无膨隆、无叩痛, 双肾下极未触及, 双侧输尿管走行区无压痛, 膀胱区叩诊鼓音。辅助检查:淋巴细胞0.7× 109/L, 白蛋白33.4 g/L, 腹部B超未见盆、腹腔积液。诊疗经过:考虑患者一般状况尚可, 采用禁食、补液、引流管负压吸引等保守治疗措施后引流液持续减少, 持续禁食、补液10 d引流量明显减少, 保持在0~2 mL/d, 恢复清淡饮食后引流量未见增加。复查血常规、血生化未见异常, 腹部B超腹、盆腔未见明显积液, 肝门部、腹主动脉旁未见明显肿大淋巴结。于2018年1月16日拔除腹膜后引流管, 无感染及淋巴瘘征象, 康复出院。
肾AML是一种常见的肾良性肿瘤, 主要由血管、平滑肌及脂肪组织构成。20%的肾AML患者同时有家族性结节性硬化症病史, 但80%为散发[5, 6]。散发性肾AML在男性中患病率为0.1%, 女性患病率为0.22%[7], 单侧多见, 无明显临床症状, 多数患者因常规超声检出而就诊, 但也可出现腹部包块、侧腹疼痛、镜下血尿及肿块破裂致大出血等临床表现[8]。
绝大多数AML在CT或MRI下可发现脂肪成分, 因此诊断并不困难, 但部分AML脂肪含量较低, 术前影像学表现不典型。近年有学者提出, 根据CT和MRI等影像学结果, AML可分为富脂型、乏脂型、无脂型三类。CT值≤ 10 HU为富脂型AML, CT值> 10 HU但MRI瘤/脾密度< 0.71或信号强度值(signal intensity index, SII)> 16.5%为乏脂型AML, CT值> 10 HU但MRI瘤/脾密度≥ 0.71或SII≤ 16.5%为无脂型AML[9](表1)。
乏脂型和无脂型肾AML在B超和平扫CT下难以与其他类型肾肿瘤相鉴别, 而当其含有丰富血管时, 在增强CT扫描下则更易被误诊为肾恶性肿瘤[2]。本例患者术前平扫CT值约44 HU, 提示可能为乏脂型AML, 虽然超声示高回声团块, 但亦有4%~13%的肾癌在超声下表现为高回声团块[10], 因此, 该患者术前影像学与恶性肾肿瘤极难鉴别。
作为一种良性肿瘤, 肾AML早在1976年被报道可伴发周围淋巴结肿大, 这是由于血管平滑肌瘤的多中心起源性, 并非肿瘤转移所导致的[11], 该现象主要在合并有结节性硬化症的病例中发现, 散发性血管平滑肌瘤伴多发淋巴结肿大者极为少见。目前文献报道的受累淋巴结主要为肾周局部淋巴结, 易误诊为肾透明细胞癌而采取肾切除术[12]。本例患者同时具备多发淋巴结肿大和CT难以发现脂肪成分这两种特点, 术前诊断困难, 因全身多淋巴结肿大而前往血液科首次就诊, 初步疑诊为“ 淋巴瘤” , 最终术后组织病理结果明确了肾AML的诊断。
| 表1 肾血管平滑肌脂肪瘤的影像学分类 Table 1 Radiologic classification of renal angiomyolipoma |
伴发淋巴结肿大的乏脂型肾AML与肾恶性肿瘤的术前鉴别尤为重要。影像学上, CT扫描中表现出液化、坏死、钙化等征象则更加提示为肾恶性肿瘤[7], 但对于极难与恶性肿瘤相鉴别的肾AML, 我们应当在采取根治性手术前利用更多方式来增加诊断的可靠性。目前推荐细针穿刺细胞学检查(fine needle aspiration cytology, FNAC)和术中冰冻组织切片, 但由于取材部位及方式的局限性, 仍存在误诊的可能[13], 因此在实际临床工作中, 我们仍应当结合患者的临床症状来确立诊断, 避免不必要的治疗。此外, 有报道称双极螺旋CT、双回声梯度回波化学位移磁共振成像等技术有望鉴别乏脂型肾AML与肾恶性肿瘤[14, 15]。
典型肾AML的处理取决于患者的临床症状和肿瘤大小、部位及其恶变倾向等。目前最推荐的治疗方案是积极随访、严密监测[16], 即便是伴有周围淋巴结肿大的肾AML, 在长期随访中也罕见转移及复发等不良事件发生[17]。当肿瘤直径> 4 cm或出现大出血等症状时, 动脉栓塞是目前推荐的一线治疗方法[18], 手术也不失为一种合理选择, 绝大多数患者可采取保肾手术以保留器官功能, 当肿瘤有恶变倾向时可选择根治性肾切除术[19, 20], 射频消融及药物靶向治疗也可供选择[21, 22]。
腹膜后淋巴瘘是泌尿系手术后较少见的并发症, 主要由术中不慎损伤淋巴管和淋巴结, 以及淋巴管堵塞引起[23]。腹膜后淋巴系统直接引流肾和睾丸淋巴液, 也是外生殖器、会阴和盆腔器官的引流通道, 泌尿系统的手术(如肾切除术、腹膜后淋巴结清扫术等)都可能造成淋巴瘘。腹膜后淋巴瘘主要表现为术后持续引流乳糜液, 目前报道的持续性引流时间从数天到数月不等[24]。本例患者术后并发淋巴瘘, 考虑可能为活检的淋巴结创面渗出淋巴液, 也可能因腹腔镜下操作不慎损伤淋巴管所致。由于患者持续较大量引流达12周之久, 结合患者原发肿瘤可累及淋巴系统、静脉系统的特性, 不除外存在淋巴管及静脉阻塞引起顽固性术后淋巴瘘的可能, 因此, 对于伴有多发淋巴结肿大的肾AML, 手术过程中我们应当加强警惕以防止损伤腹膜后淋巴系统, 术后也应当注意观察患者是否合并淋巴瘘。
临床症状和体征是术后淋巴瘘的诊断要点, 对于放置引流管的患者, 持续引流乳糜状液体是主要表现, 也可有腹胀、腹痛、恶心、呕吐等主诉[25]。对于出现上述表现的患者, 可行引流液成分分析以明确诊断。典型瘘液为碱性、无特殊气味的牛奶状或乳糜状液体, 细菌培养阴性, 其甘油三酯含量是血浆的2~8倍, 比重及蛋白质含量均高于血浆。显微镜下引流液涂片苏丹红染色可发现脂肪滴, 亦可见以淋巴细胞为主的大量白细胞[26]。对于穿刺获得瘘液困难或者需要明确淋巴瘘部位者, 亦可采用超声、CT、淋巴管造影、淋巴结核素显像等手段协助诊治[27, 28]。
淋巴瘘造成的持续性蛋白和淋巴细胞丢失是患者术后恢复的不利因素, 而对于腹膜后间隙这一狭窄腔隙, 淋巴瘘易引起患者一系列的临床症状, 故而及早采取处理措施是必要的。Aalami等[29]报道了156例腹腔淋巴瘘患者, 有67%经保守治疗后痊愈, 保守治疗是术后淋巴瘘的首选方案。在保证通畅引流防止腹膜后淋巴液积聚的前提下, 限制饮食是处理淋巴瘘最关键的步骤。对于术后淋巴瘘患者, 宜采用高蛋白、低长链甘油三酯(long-chain trigly-ceride, LCT)、正常中链甘油三酯(medium-chain triglyceride, MCT)饮食。负压吸引可局限淋巴液, 促进周围组织愈合粘连, 有助于瘘口闭合, 但应当采用低压负压吸引, 以避免过度吸引造成瘘口扩大。对于难治性及复发性淋巴瘘, 全肠外营养不仅可以减少淋巴液漏出, 也可补充营养、防止负氮平衡以促进恢复。生长抑素、腔内注射红霉素或高渗性葡萄糖等也可用于治疗顽固性淋巴瘘[30]。如出现脱水危及患者生命等情况, 再次手术结扎缝合瘘口是迫不得已的选择, 但再次手术可能存在更高的风险, 需要经过慎重评估和权衡利弊后做出选择。
综上所述, 伴发淋巴结肿大的乏脂型肾AML在诊断方面应该引起我们的重视与警惕, 明确诊断、规范治疗和术后管理有赖于临床工作者增加对该病临床特点的认识和进一步研究。我们应当尽量避免因对该病认识不足而造成不必要的手术负担和器官损失, 以帮助患者实现最大获益。
(本文编辑:任英慧)
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|


