SUMMARY Peritoneal dialysis (PD)-related peritonitis is recognized as a common complication of peritoneal dialysis. Eosinophilic peritonitis is a rare type of non-infection PD-related peritonitis. Eosinophilic peritonitis in continuous ambulatory peritoneal dialysis (CAPD) patients was first reported in 1967. The cause of eosinophilic peritonitis is obscure, however it may be related to some etiologies: (1) hypersensitivity to PD materials, including catheter or dialysate; (2) bacteria, fungal or mycobacterium tuberculosis infection. Clinical investigations include asymptomatic cloudy PD effluent, fever, abdominal pain and eosinophil count elevate in PD effluent. Eosinophilic peritonitis is usually mild and self-limited. With the development of PD, more eosinophilic peritonitis cases and researches were reported. Here, we report a patient on CAPD with eosinophilic peritonitis. A 71-year-old female patient developed end-stage renal disease for 4 years and underwent CAPD (2 000 mL of 1.5% dialysis solution with four exchanges daily) for 5 months. With a history of unclean food, she was hospitalized for complaints of diarrhea, fever and cloudy peritoneal effluent for 10 days. Dialysis effluent showed an elevated white blood cell (WBC) count of 1 980 cell/mm3, with 60% polymorphonuclear cells. She was diagnosed as PD-related peritonitis, and therapy was initiated with intraperitoneal ceftazidime 1 g once a day and vancomycin 500 mg every other day. She was admitted to the hospital as the symptoms were not relieved. Her peripheral blood cell count showed a total WBC count of 6 940 cells/mm3, 36.8% eosinophil. Her PD effluent analysis showed turbidity, total WBC count of 1 480 cells/mm3, and 83% polymorphonuclear cells. Her dialysate bacteria culture, fungus culture, polymerase chain reaction for Mycobacterium tuberculosis (TB-PCR), acid-fast stain were all negative. On admission day 4, the treatments were changed to levofloxacin 200 mg once a day and vancomycin 500 mg every other day. After two weeks of antibiotics treatment, patient’s symptoms were not completely improved and her dialysis effluent remained cloudy. Her blood eosinophil count elevated to 36.8%,eosinophil proportion in PD effluent>90% and PD effluent pathological findings showed eosinophil>90%. Eosinophilic peritonitis was diagnosed and a decision was made to give loratadine daily dose of 10 mg orally. The possible reasons might be the patient’s allergy to some components of PD solution or connection systems in the beginning of PD, and this bacterial peritonitis episode, as well as the application of vancomycin, might lead to the fact that eosinophilic peritonitis acutely developed. For there was no improvement in clinical symptoms, loratadine was stopped, and the patient was discharged 18 days later, and received follow-up closely. Two months later, eosinophil count in blood and PD fluid decreased to normal range with no symptom. This case reminds us that in any PD-related peritonitis patient with prolonged symptoms after appropriate antibiotic therapy, and typical clinical symptoms, the diagnosis of eosinophilic peritonitis should be considered. For the count and per-centage of eosinophils are not routinely reported in most laboratories, doctors need to contact the department of laboratory and the department of pathology, to confirm the cell count and proportion of eosinophils in dialysis effluent, so as to make the definite diagnosis, which can not only avoid antibiotics overuse, but also avoid antibiotics-induced eosinophilic peritonitis (such as vancomycin).
腹膜透析相关性腹膜炎是腹膜透析(peritoneal dialysis)重要且常见的并发症。嗜酸粒细胞性腹膜炎(eosinophilic peritonitis)是一种少见的特殊类型的非感染性腹膜透析相关性腹膜炎, 目前病因未明, 通常与患者对透析系统的组成成分(如管路或透析液)过敏, 或与细菌、真菌、结核等感染相关, 临床表现为无症状性腹膜透析液(简称腹透液)混浊、低热、轻度腹痛、腹透液中嗜酸性粒细胞比例增高, 多具有自限性。腹膜透析相关嗜酸粒细胞性腹膜炎在1967年由Lee等[1]首次报道。近年来随着腹膜透析技术的推广及发展, 嗜酸粒细胞性腹膜炎的病例报道有所增加, 但国内鲜有报道。现将我院诊治的1例腹膜透析相关嗜酸粒细胞性腹膜炎患者的情况进行总结报道如下, 并结合病例进行文献复习。
患者女性, 71岁, 主因“ 血肌酐升高4年, 腹膜透析5月余, 透析液混浊10日” 入院。患者4年前因“ 冠心病、心力衰竭” 伴双下肢水肿, 血生化检查血肌酐124 μ mol/L, 尿蛋白1+, 24小时尿蛋白 2.61 g(尿量3 650 mL), 血红蛋白103 g/L, 诊断为“ 慢性肾病(chronic kidney disease, CKD)3期” , 给予纠正贫血、控制血糖、血压、对症治疗, 但血肌酐进行性升高。
患者5个多月前因“ CKD 5期、高钾血症” 行腹膜透析置管术, 开始行持续性不卧床腹膜透析(continuous ambulatory peritoneal dialysis, CAPD)治疗, 腹透方案:每天1.5%(质量分数)腹透液2 000 mL× 4次, 白天存腹5 h, 夜间存腹9 h, 每日超滤量约300 mL, 尿量1 000 mL, 并规律行降血压、降血糖、纠正贫血等治疗。患者10 d前因不洁饮食后腹泻4~5次, 次日发现透析液浑浊, 其内偶见絮状物, 每日超滤量减少至50 mL, 低热, 体温最高37.9 ℃, 无腹痛。7 d前于我院查腹水常规示白细胞1 980/μ L, 分叶核细胞60%, 考虑“ 腹透相关性腹膜炎” , 予头孢他啶1 g每天1次+万古霉素500 mg每3天1次加入透析液存腹治疗。患者入院前3 d体温恢复正常, 但腹透液仍浑浊, 复查腹水常规示白细胞1 480/μ L, 分叶核细胞83%, 收入院进一步诊治。
患者自发病以来, 精神、饮食、睡眠欠佳, 尿量每日1 300 mL左右, 体重无明显变化。既往患2型糖尿病21年, 诊断“ 糖尿病肾病、糖尿病周围神经病变、糖尿病视网膜病变” 3年。高血压病史6年, 冠心病、陈旧性心肌梗死病史4年, 腔隙性脑梗塞病史3年, 周围动脉硬化症病史2年, 无结核病史及密切接触史, 无药物过敏史, 对海鲜过敏, 无家族性遗传病, 无冠心病早发家族史, 无高血压、糖尿病家族史。
入院体格检查:体温36.0 ℃, 脉搏70次/min, 呼吸18次/min, 血压110/60 mmHg(1 mmHg=0.133 kPa)。心、肺未见异常, 腹软, 无压痛、反跳痛, 腹部可见腹膜透析管, 移动性浊音(+), 双下肢轻度可凹陷性水肿。
入院后实验室检查:血白细胞6.94× 109/L, 嗜酸性粒细胞36.8%, 嗜酸性粒细胞绝对值2.55× 109/L, 血红蛋白82 g/L, 血小板187× 109/L, 谷丙转氨酶14 U/L, 谷草转氨酶20 U/L, 总蛋白46.3 g/L, 白蛋白25.1 g/L, 血清肌酐433 μ mol/L。腹透液常规检查:外观混浊, 比重1.010, 李瓦他试验(Rivalta test)(+), 总细胞2 210/μ L, 白细胞1 480 /μ L, 中性分叶核粒细胞83%, 单核细胞17%。住院期间多次腹透液细菌培养阴性, 多次腹透液细菌涂片未见细菌、真菌孢子及菌丝, 多次腹透液抗酸染色阴性、T细胞斑点试验(T-SPOT)阴性、结核分枝杆菌扩增荧光检测阴性, 血IgE正常(6.57 IU/mL)。
初步诊断:腹膜透析相关性腹膜炎, 维持性腹膜透析, 糖尿病肾病可能, 糖尿病, 高血压, 陈旧性心肌梗死、腔隙性脑梗塞。
治疗方案:经验性腹腔内灌注头孢他啶1 g每天1次+万古霉素500 mg隔天1次, 治疗一周, 患者仍间断低热, 腹透液白细胞总数仍> 1 000/μ L, 考虑细菌耐药可能, 入院4 d后调整为盐酸左氧氟沙星200 mg每天1次+万古霉素500 mg隔天1次腹腔内用药治疗, 腹透液白细胞总数稍下降, 但仍维持在500/μ L左右(图1)。经两周抗生素治疗后, 患者临床表现无明显改善, 仍间断低热, 腹透液浑浊, 腹痛不明显, 超滤无明显减少, 但血嗜酸性粒细胞进一步明显升高, 最高36.8%(图2)。回顾患者开始腹膜透析的5个月来, 血嗜酸性粒细胞进行性增高, 此次腹膜炎后更为明显, 故请检验科加做腹透液中嗜酸性粒细胞计数, 发现嗜酸性粒细胞> 90%, 请病理科行腹透液嗜酸染色, 结果见大量嗜酸性粒细胞(> 90%)、个别退变的淋巴细胞、中性粒细胞、间皮细胞及红细胞。至此, 考虑患者为“ 细菌性腹膜炎抗感染治疗后合并嗜酸粒细胞性腹膜炎” , 于入院第8天加用开瑞坦10 mg每天1次口服, 入院第19天停用抗生素, 开瑞坦持续应用18 d, 因血嗜酸性粒细胞比例及腹透液白细胞总数、嗜酸性粒细胞比例无明显下降, 结合患者无临床不适症状, 超滤未受影响, 停药出院。出院时患者血嗜酸性粒细胞比例32%, 腹透液白细胞总数940/μ L, 多形核白细胞70%。
 | 图1 腹膜透析液常规白细胞总数及中性分叶核粒细胞比例变化Figure 1 Total WBC count and percentage of polymorphonuclear cells in PD effluentqd, once a day; qod, every other day; WBC, white blood cell; PD, peritoneal dialysis. |
后续随访:出院后患者未再应用抗生素及抗过敏药物, 监测腹透液常规及血嗜酸性粒细胞比例, 腹透液白细胞总数逐渐下降, 出院后2.5个月降低至28/μ L(图3), 血嗜酸性粒细胞比例亦逐渐下降, 出院后3.5个月降至正常即5.5%, 出院后12个月腹透液白细胞总数94/μ L, 血嗜酸性粒细胞曾经一过性升高至55%, 未做处理, 出院后14个月, 白细胞54/μ L, 嗜酸性粒细胞恢复至0.1%, 完全正常, 且患者无发热、腹痛、腹透液浑浊等临床表现, 至今随访3年余, 血嗜酸性粒细胞比例正常, 腹透液中白细胞总数、多形核白细胞、嗜酸性粒细胞比例均正常。
腹膜透析相关性嗜酸粒细胞性腹膜炎是一种特殊类型的非感染性腹膜炎, 或称“ 无菌性腹膜炎” 。嗜酸粒细胞性腹膜炎一般在开始腹膜透析的3个月内发生[2], 也可发生在维持性透析1~2年甚至更久的患者[3, 4]。临床表现为无症状性腹透液混浊、低热、轻度腹痛、腹透液中嗜酸粒细胞比例增高。组织病理检查显示腹膜血管扩张伴大量嗜酸细胞[5]。诊断标准:符合腹膜透析相关性腹膜炎的患者具备腹膜透析液中嗜酸粒细胞数绝对值> 100/μ L或嗜酸粒细胞数占白细胞总数> 10%[6]。有学者将腹膜透析患者透析液中嗜酸粒细胞数占白细胞总数> 10%, 但无腹痛及透析液混浊等临床表现视为腹膜嗜酸粒细胞增多症[6]。
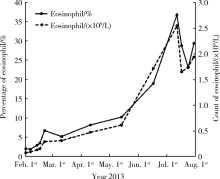 | 图2 患者自开始腹膜透析后血嗜酸性粒细胞计数及比例变化Figure 2 Count and percentage of eosinophil in blood after initiation of peritoneal dialysis |
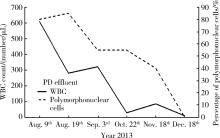 | 图3 随访患者腹膜透析液常规白细胞总数及中性分叶核粒细胞比例变化Figure 3 Total WBC count and percentage of polymorphonuclear cells in PD effluent of the follow-up patientsWBC, white blood cell; PD, peritoneal dialysis. |
嗜酸粒细胞性腹膜炎目前发病原因未明, 认为可能的原因包括:(1)对腹膜透析材料或组分发生过敏反应, 如透析液、导管、麦考糊精等, 这种情况多发生在腹膜透析开始后的早期, 多在3个月内。(2)化学刺激、空气、尿毒症诱发。(3)继发于感染性腹膜炎, 尤其是真菌性腹膜炎、结核性腹膜炎[7]。透析人群因细胞免疫容易低下, 因此有极高风险发生结核分枝杆菌感染[8]。结核分枝杆菌感染可伴随嗜酸粒细胞增多, 后者参与结核分枝杆菌感染的炎症反应, 其过氧化物酶可以诱导结核分枝杆菌表面改变、死亡[9]。曾有报道, CAPD合并嗜酸粒细胞腹膜炎的病例, 腹膜透析液抗酸染色、分枝杆菌培养皆阴性, 最终经PCR证实为结核分枝杆菌感染, 从而证实嗜酸粒细胞性腹膜炎可由结核分枝杆菌感染所引起, 经抗结核治疗后患者嗜酸粒细胞性腹膜炎亦明显缓解[7]。(4)腹腔内应用药物所致。已有病例报道, 腹膜透析患者应用万古霉素治疗细菌性腹膜炎后, 嗜酸粒细胞比例分别在应用万古霉素后第10天、12天时达到高峰(84%、69%)[3, 10], 可能的机制包括过敏、药物超敏综合征(drug rash with eosinophilia and systemic symptoms, DRESS)、延迟型细胞免疫[11, 12, 13], 其他抗生素(如庆大霉素、链激酶、头孢类抗生素、哌拉西林)也有报道可以引起嗜酸粒细胞性腹膜炎[14]。
关于嗜酸粒细胞性腹膜炎对腹膜功能的影响报道不尽相同。有学者通过前瞻性研究, 随访9个月后发现, 长期存在的腹透液中嗜酸性粒细胞增多并未明显影响腹膜功能, 包括肌酐清除率、尿素清除率等[15], 但是也有学者研究发现, 在嗜酸粒细胞性腹膜炎时, 患者外周血嗜酸性粒细胞可以升高, 腹膜组织病理可见肥大细胞、嗜酸性粒细胞浸润, 因炎症介质增多引起腹膜血管扩张、通透性增加, 可造成腹膜转运增加[5]。亦有关于CAPD患者持续反复发生嗜酸粒细胞性腹膜炎, 最终演变成包裹性硬化性腹膜炎的病例报道[16]。本例患者住院期间及出院后随访, 腹膜功能未受到影响, 超滤量、透析充分性与发病前相比均无明显改变。由于关于此病对腹膜功能影响的研究较少, 仍需进一步探讨。
嗜酸粒细胞性腹膜炎多为自限性, 可以在几周后自发缓解, 或者在去除相关致病因素后缓解, 如持续不缓解, 治疗可选择糖皮质激素[16]、H1受体拮抗剂(苯海拉明[17]、酮替芬[18]), 不必使用抗生素。近年有文献报道, 白三烯受体拮抗剂(montelukast, 孟鲁司特)也是一种新的治疗选择[19]。白三烯是由白细胞、肥大细胞、巨噬细胞经活化5-脂氧合酶途径所产生的促炎因子, 参与嗜酸粒细胞的趋化作用[20]。孟鲁斯特是一种白三烯受体拮抗剂, 常用于儿童过敏性疾病, 但目前在肾功能不全患者中的药物动力学研究尚不充分, 仍然需要更多病例、循证医学证据支持。
本例腹膜透析患者此次发病腹透液浑浊, 伴低热, 腹透液常规白细胞总数明显升高, 且中性分叶核粒细胞为83%。腹膜透析相关性腹膜炎的诊断标准为:(1)腹痛, 腹透液浑浊, 伴或不伴发热; (2)腹透液常规白细胞> 100/μ L, 中性分叶核粒细胞> 50%; (3)腹透液培养有病原微生物生长; 具备上述3项中的2项或以上, 即可确定诊断, 因此本例患者“ 腹膜透析相关性腹膜炎” 诊断成立。在2周抗生素治疗过程中, 患者再次间断低热, 腹透液持续浑浊, 多次查细菌涂片及培养均为阴性, 需要鉴别:(1)真菌性腹膜炎:多有明显腹痛, 腹部压痛、反跳痛, 涂片可见真菌孢子或菌丝, 本例患者无上述表现, 故不支持。(2)结核性腹膜炎:多在透析后12个月内发生, 症状常不典型, 抗生素治疗无效, 症状迁延, 细菌培养阴性的复发性腹膜炎时疑诊此病。可表现为发热、腹痛、腹透液混浊, 腹透液常规检查以中性粒细胞为主, 腹透液涂片可见抗酸杆菌, 诊断金标准为腹透液培养抗酸杆菌阳性。本例患者应用抗生素治疗后症状迁延, 腹透液常规以多核细胞为主, 但无明显腹痛, 应用喹诺酮类抗生素后仍有低热及腹透液白细胞增多, 查T-SPOT、结核分枝杆菌扩增荧光检测均阴性, 考虑此病可能不大。(3)非感染性腹膜炎:患者病程中出现血嗜酸粒细胞比例明显升高, 最高达36.8%, 化验腹透液中嗜酸细胞比例明显升高。回顾患者既往病史, 在开始腹膜透析3周左右, 血嗜酸粒细胞比例即开始上升, 嗜酸粒细胞性腹膜炎诊断成立, 原因可能与患者对腹透液或腹透管路某些成分过敏有关, 而本次不洁饮食腹泻后出现感染性腹膜炎以及万古霉素的应用, 可能是诱导嗜酸粒细胞性腹膜炎急剧发生并加重的诱因。随着透析时间延长, 未予任何药物干预, 患者腹膜炎症状和体征逐步好转, 呈现自限性, 支持嗜酸粒细胞性腹膜炎的诊断。
综上所述, 嗜酸粒细胞性腹膜炎在腹膜透析患者中并不常见, 临床症状很轻, 确诊需要依据腹透液常规白细胞计数、嗜酸性粒细胞计数及比例, 一般无需使用抗生素, 具有自限性。对于腹膜透析相关性腹膜炎, 当给予规范的抗生素治疗后, 腹膜炎仍迁延且临床症状不典型的患者, 要警惕是否存在嗜酸粒细胞性腹膜炎。由于大多数实验室并不常规报告嗜酸性粒细胞的计数及百分比, 只笼统地报告多形核白细胞的总数, 因此容易漏诊, 这时需要联系检验科与病理科及时协助明确诊断, 不但可以避免抗生素滥用, 也可避免因抗生素(如万古霉素)引起患者嗜酸粒细胞性腹膜炎病情加重的可能。
(本文编辑:任英慧)
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|


