目的: 观察米诺环素对脊髓背角胶状质(substantia gelatinosa, SG)区神经元突触传递的影响,以阐明其在甲醛炎性痛中的作用机制。方法: 行为学和免疫组织化学实验:将30只3~5周龄雄性SD大鼠,随机分为对照组(8只)、模型组(8只)、生理盐水模型组(6只)和米诺环素模型组(8只)。对照组采用右后足背皮下注射生理盐水,模型组(甲醛炎性痛模型)采用右后足背皮下注射5%(体积分数)甲醛溶液,生理盐水模型组和米诺环素模型组分别在模型制备前1 h腹腔注射生理盐水和米诺环素。记录4组大鼠足背皮下注射生理盐水或甲醛溶液后1 h内每5 min 缩足和舔爪的时间,共记录1 h。痛行为学记录结束1 h后,行4%多聚甲醛心脏灌流取脊髓组织,以免疫组化实验的方法观察脊髓背角c-Fos蛋白的表达。电生理实验:选取26只3~5周龄雄性SD大鼠制作离体脊髓纵切片,每只大鼠随机选取2~5个神经元进行全细胞膜片钳记录,分别记录米诺环素、氟代柠檬酸和多西环素对SG神经元的自发性兴奋性突触后电流(spontaneous excitatory postsynaptic currents, sEPSCs)或自发性抑制性突触后电流(spontaneous inhibitory postsynaptic currents, sIPSCs)的作用。结果: 模型组与对照组比较,右侧缩足和舔爪等炎性痛行为及脊髓背角c-Fos蛋白表达显著增加;腹腔注射米诺环素可显著减轻大鼠第二相的炎性痛行为 ( t= 2.957, P<0.05), 并减少脊髓背角浅层(Ⅰ~Ⅱ)和深层(Ⅲ~Ⅳ)c-Fos蛋白的表达( tⅠ-Ⅱ= 3.912, tⅢ-Ⅳ= 2.630, P<0.05)。米诺环素显著增加SG神经元的sIPSCs的频率至用药前的220%±10%( P<0.05),但对sEPSCs的频率(100%±1%, t=0.112, P=0.951)和幅度(98%±1%, t=0.273, P=0.167)、sIPSCs的幅度(105%±3%, t=0.568, P=0.058)均无显著影响。氟代柠檬酸和多西环素对sIPSCs的频率[分别为:(99%±1%, t=0.366, P=0.099);(102%±1%, t=0.184, P=0.146)]和幅度[分别为:(98%±1%, t=0.208, P=0.253);(99%±1%, t=0.129, P=0.552)] 均无显著影响。结论: 米诺环素可抑制甲醛炎性痛及减少脊髓背角c-Fos蛋白的表达,这些效应与其增强SG神经元的抑制性突触传递有关,而与其抑制小胶质细胞的激活及抗生素的效应无关。
*These authors contributed equally to this work
Objective: To unravel the underlying mechanism of minocycline in formalin-induced inflammatory pain, and to investigate the effects of minocycline on synaptic transmission in substantia gela-tinosa (SG) neurons of rat spinal dorsal horn.Methods: Behavioral and immunohistochemistry experiments: 30 male Sprague-Dawley (SD) rats (3-5 weeks old) were randomly assigned to control ( n=8 rats), model ( n=8 rats), saline treatment model ( n=6 rats) and minocycline treatment model ( n=8 rats) groups. The control group was subcutaneously injected with normal saline on the right hindpaws. Acute inflammatory pain model was established by injecting 5% (volume fraction) formalin into the right hindpaws. The rats in the latter two groups received intraperitoneal injection of saline and minocycline 1 h before the formalin injection, respectively. The time of licking and lifting was recorded every 5 min within 1 h after the subcutaneous injection of normal saline or formalin for all the groups, which was continuously recorded for 1 h. One hour after the pain behavioral recording, the spinal cord tissue was removed following transcardial perfusion of 4% paraformaldehyde. The expression of c-Fos protein in spinal dorsal horn was observed by immunohistochemistry. Electrophysiological experiment: In vitro whole-cell patch-clamp recordings were performed in spinal cord parasagittal slices obtained from 26 male SD rats (3-5 weeks old). Two to five neurons were randomly selected from each rat for patch-clamp recording. the effects of minocycline, fluorocitrate and doxycycline on spontaneous excitatory postsynaptic currents (sEPSCs) or spontaneous inhibitory postsynaptic currents (sIPSCs) of SG neurons were investigated.Results: Compared with the control group, both the licking and lifting time and the expression of c-Fos protein in ipsilateral spinal dorsal horn of the model group were significantly increased. Intraperitoneal injection of minocycline largely attenuated the second phase of formalin-induced pain responses ( t=2.957, P<0.05). Moreover, c-Fos protein expression was also dramatically reduced in both the superficial la-mina (Ⅰ-Ⅱ) and deep lamina (Ⅲ-Ⅳ) of spinal dorsal horn ( tⅠ-Ⅱ = 3.912, tⅢ-Ⅳ = 2.630, P<0.05). On the other side, bath application of minocycline significantly increased the sIPSCs frequency to 220%±10% ( P<0.05) of the control but did not affect the frequency (100%±1%, t = 0.112, P=0.951) and amplitude (98%±1%, t=0.273, P=0.167) of sEPSCs and the amplitude (105%±3%, t = 0.568, P = 0.058) of sIPSCs. However, fluorocitrate and doxycycline had no effect on the frequency [ (99% ± 1%, t = 0.366, P = 0.099); (102% ± 1%, t = 0.184, P = 0.146), respectively] and amplitude [ (98% ± 1%, t = 0.208, P = 0.253); (99% ± 1%, t = 0.129, P=0.552), respectively] of sIPSCs.Conclusion: Minocycline can inhibit formalin-induced inflammatory pain and the expression of c-Fos protein in spinal dorsal horn. These effects are probably due to its enhancement in inhibitory synaptic transmission of SG neurons but not its effect on microglial activation or antibiotic action.
米诺环素(minocycline)是一种二代半合成四环素, 临床上主要用于治疗浅表性化脓性感染(如痤疮、毛囊炎)以及淋病、沙眼衣原体所致的性传播疾病等, 此外, 也常被用作小胶质细胞激活的抑制剂。近年来其镇痛效应引起了学者们的广泛关注, 米诺环素不仅可显著缓解多种动物模型的病理性疼痛, 如神经病理性痛[1]、炎性痛[2]、癌性痛[3]、糖尿病及化疗所致疼痛等[4, 5, 6], 而且可增强吗啡等药物的镇痛效应[7, 8, 9, 10, 11]。以往的研究多认为米诺环素的镇痛效应与其抑制小胶质细胞激活, 进而减少炎性介质的释放有关[12], 近来研究表明米诺环素可直接作用于神经元, 如抑制背根神经元的钠电流或减少脊髓背角神经元的动作电位放电频率, 降低神经元的兴奋性[13, 14, 15]。脊髓背角胶状质(substantia gelatinosa, SG)区位于脊髓背角Ⅱ 层, 是伤害性信息向中枢传递的重要门户, 米诺环素是否能直接影响该区神经元的突触传递即微小突触电流的大小目前尚不清楚。c-Fos蛋白是一种对外界刺激发生快速反应的蛋白, 是伤害性刺激的标志物, 研究发现大鼠甲醛致炎后1~2 h可诱导中枢神经系统特定区域内c-Fos 基因大量表达[16]。根据以上研究, 我们推测米诺环素有可能参与调控SG神经元的电活动, 为进一步揭示米诺环素镇痛的中枢机制, 本研究首先观察米诺环素对炎性痛模型大鼠行为学及脊髓背角c-Fos蛋白表达的影响, 而后观察米诺环素及其类似物对SG神经元的兴奋性及抑制性突触电流的作用, 以期为米诺环素用于临床疼痛治疗提供新的理论依据。
1.1行为学实验
1.1.1 甲醛炎性痛模型 参照Dickenson等[17]方法, 选取3~5周龄雄性SD大鼠(购于江西中医药大学动物中心), 采用右后足背皮下注射5%(体积分数)甲醛溶液(购自南昌雨露实验器材有限公司)50 μ L的方法建立急性炎性痛模型, 注射足出现缩足、红肿、舔爪等自发痛行为表示建模成功。
1.1.2 实验分组 SD大鼠30只, 随机分为4组, 对照组:右后足背皮下注射生理盐水50 μ L, n=8; 模型组:右后足背皮下注射5%甲醛溶液, n=8; 生理盐水模型组:腹腔注射1 mL生理盐水, 1 h后右后足背皮下注射5%甲醛溶液, n=6; 米诺环素模型组:腹腔注射米诺环素(45 mg/kg[13]溶于1 mL生理盐水, 米诺环素购自美国Sigma公司), 1 h后右后足背皮下注射5%甲醛, n=8。
1.1.3 痛行为学观察 将大鼠放入透明观察盒中, 待动物熟悉环境后, 各组分别接受皮下注射和/或腹腔注射。皮下注射后即刻将大鼠放入透明盒中, 模型组大鼠均有不同程度特征性缩足、颤抖、咬爪、舔爪等炎性痛行为, 本研究采用Abbott等[18]推荐的以缩足和舔爪时间作为行为学反应的指标, 分析每5 min 内缩足和舔爪时间之和, 共1 h。
1.1.4 c-Fos蛋白表达细胞计数 采用免疫组织化学染色, 各组大鼠行为学观察结束后1 h, 腹腔注射乌拉坦(1.5 g/kg)麻醉, 先后用生理盐水(100 mL)和含4%多聚甲醛的0.1 mmol/L磷酸缓冲液(400 mL)行心脏灌流, 取出脊髓腰骶膨大部放入相同4%多聚甲醛溶液固定6 h, 而后放入30%蔗糖溶液3 d(4 ℃), 每只大鼠的脊髓以冰冻切片机切取4片横向切片, 片厚30 μ m, 用两步法进行常规漂染, 脊髓切片用小鼠单克隆一抗c-Fos(1 :1 000, 购自英国Abcam公司)4 ℃孵育24 h后用磷酸盐缓冲液清洗3遍, 再用对应的小鼠二抗Alexa Fluor 647(1 :400, 购自美国Invitrogen 公司)4 ℃孵育过夜, 共聚焦显微镜(CM1950, 购自德国Leica公司)呈像, 观察脊髓背角c-Fos蛋白的表达, 参照Presley等[19]方法对每只大鼠脊髓背角浅层(Ⅰ ~Ⅱ )和深层(Ⅲ ~Ⅳ )中c-Fos蛋白表达阳性细胞计数并取其平均值。
1.2.1 试剂和溶液 行心脏灌流时使用解剖液, 其配方为240 mmol/L 蔗糖, 2.5 mmol/L KCl, 0.5 mmol/L CaCl2· 2H2O, 3.5 mmol/L MgCl2· 6H2O, 1.25 mmol/L NaH2PO4· 2H2O, 25 mmol/L NaHCO3, 0.4 mmol/L L-抗坏血酸钠, 2 mmol/L 丙酮酸钠, pH=7.4, 310 毫渗透摩尔; 持续灌流脊髓切片时使用Krebs液, 其配方为117 mmol/L NaCl, 3.6 mmol/L KCl, 2.5 mmol/L CaCl2· 2H2O, 1.2 mmol/L MgCl2· 6H2O, 1.2 mmol/L NaH2PO4· 2H2O, 25 mmol/L NaHCO3, 11 mmol/L蔗糖, 0.4 mmol/L L-抗坏血酸钠, 2 mmol/L丙酮酸钠, pH=7.4, 310毫渗透摩尔; 记录sEPSCs时使用钾电极内液, 其配方为130 mmol/L K-gluconate, 5 mmol/L KCl, 10 mmol/L 磷酸肌酸, 0.5 mmol/L EGTA, 10 mmol/L HEPES, 4 mmol/L Mg-ATP, 0.3 mmol/L Li-GTP, pH=7.2, 300 毫渗透摩尔; 记录sIPSCs时使用铯电极内液, 其配方为92 mmol/L CsMeSO4, 43 mmol/L CsCl, 10 mmol/L 磷酸肌酸, 0.5 mmol/L EGTA, 10 mmol/L HEPES, 4 mmol/L Mg-ATP, 0.3 mmol/L Li-GTP, 5 mmol/L tetraethylammonium (TEA)-Cl, pH=7.2, 300 毫渗透摩尔。米诺环素、氟代柠檬酸(fluorocitrate)和多西环素(doxycycline)分别用去离子水配置成1 :1 000的储备液, 分装后保存于 -20 ℃, 使用时以Krebs液配置成所需终浓度。多西环素购自英国Tocris公司, 其余试剂均购自美国Sigma公司。在重力作用下用三通管转换灌流液的方式进行给药。
1.2.2 脊髓切片的制备 参照我们实验室之前的方法[15], 选取26只3~5周龄的雄性SD大鼠, 腹腔注射乌拉坦(1.5 g/kg)麻醉后, 用预先充混合气(95%O2 + 5%CO2)的 0~4 ℃ 解剖液行心脏灌流, 同时迅速分离脊柱旁肌肉、离断两侧肋骨, 取出腰骶段脊柱并完全浸入解剖液中。以腹侧入路打开椎板, 取出腰骶段脊髓。剪去脊髓两侧附着的神经根并剥离脊髓表面的硬脊膜、软脊膜, 放置于琼脂块上, 用振动切片机(VT1000S, 购自德国Leica公司)切取300 μ m厚纵切片。将脊髓切片置于32 ℃充氧的Krebs液中孵育至少30 min。
1.2.3 全细胞膜片钳记录 脊髓切片孵育结束后, 将其移入记录槽中, 室温(22~25 ℃)下用充以混合气的Krebs液持续灌流, 流速为2~4 mL/min。记录电极由玻璃微电极经电极拉制仪(P-97, 购自美国Sutter Instrument公司)拉制而成, 充灌电极内液后阻抗为3~5 MΩ 。在钳制电压为 -70 mV时记录自发性兴奋性突触后电流(spontaneous excitatory postsynaptic currents, sEPSCs)一致; 在钳制电压为0 mV 时记录自发性抑制性突触后电流(spontaneous inhibitory postsynaptic currents, sIPSCs)。因为sIPSCs的反转电压在 -70 mV附近, 所以 -70 mV记录到的sEPSCs不含有sIPSCs的成分。与此类似, 因为sEPSCs的反转电压在0 mV附近, 所以0 mV记录到的sIPSCs不含有sEPSCs的成分。研究米诺环素对SG神经元sEPSCs和sIPSCs的作用, 数据分别来自5只SD大鼠9个神经元和12只SD大鼠的17个神经元。研究氟代柠檬酸和多西环素对SG神经元sIPSCs的作用, 数据分别来自5只SD大鼠15个神经元和4只SD大鼠的12个神经元。所有实验结果的记录均在全细胞膜片钳封接后5~10 min、电流稳定之后进行。细胞电信号的采集采用德国HEKA公司的EPC-10放大器及Patch master软件记录。
sEPSCs和sIPSCs的频率及幅度采用Mini Ana-lysis 软件(version 6.0.3, 购自美国Synaptosoft公司)进行分析。图表绘制采用GraphPad Prism 5.0, 统计学分析采用SPSS 17.0统计软件。所有数据均以均数± 标准误表示, n 为动物或所记录神经元或脊髓片的目数。用药前后差异显著性采用配对样本t检验, 两组独立样本则采用两独立样本t检验。以P< 0.05为差异有统计学意义。
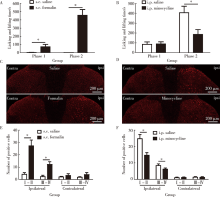
皮下注射5%甲醛溶液后炎性痛行为出现典型的两相:0~5 min为第一相(Phase 1), 经5~10 min的间歇期后出现第二相(Phase 2, 20~40 min)。模型组大鼠均立即出现缩足和舔爪等炎性痛行为(图1A、B), 而对照组大鼠仅在前10 min偶见缩足和舔爪反应(图1A)。如图1B所示, 米诺环素模型组与生理盐水模型组在第一相的痛行为累计时间差异无统计学意义(t=0.111, P = 0.913), 但米诺环素可显著减轻第二相的痛行为(t = 2.957, P < 0.05)。甲醛炎性痛大鼠非术侧仅有少量c-Fos蛋白表达, 而术侧脊髓背角各层均有c-Fos蛋白大量表达, 尤其是背角浅层(图1C、D、E、F)。米诺环素模型组术侧脊髓背角浅层和深层的c-Fos蛋白的表达与生理盐水模型组术侧相比, 均显著减少(tⅠ -Ⅱ = 3.912, tⅢ -Ⅳ = 2.630, P < 0.05, 图1D、F)。
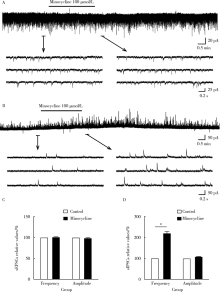
如图2所示, 以Krebs液灌流时所得sEPSCs为对照, 100 μ mol/L 米诺环素灌流3 min后 sEPSCs的频率(100%± 1%, t=0.112, P=0.951)和幅度(98%± 1%, t=0.273, P=0.167)无显著变化(图2A、C), 但sIPSCs的频率显著增加(220%± 10%, t=2.271, P< 0.05), 幅度变化不明显(105%± 3%, t=0.568, P=0.058, 图2B、D)。
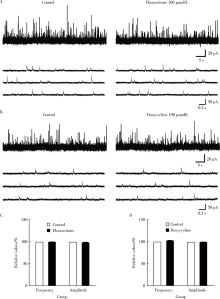
如图3所示, 氟代柠檬酸对SG神经元sIPSCs的频率(99%± 1%, t = 0.366, P=0.099)和幅度(98%± 1%, t = 0.208, P=0.253)均无明显影响(图3A、C)。接下来我们在同一神经元中先灌流氟代柠檬酸, 以阻断小胶质细胞的激活, 并记录此时的sIPSCs作为对照, 然后再灌流100 μ mol/L 米诺环素并观察其对sIPSCs的影响。结果表明米诺环素后仍可显著增加sIPSCs的频率, 与单用米诺环素组的结果差异无统计学意义。100 μ mol/L多西环素灌流3 min后sIPSCs的频率(102%± 1%, t =0.184, P=0.146)和幅度(99%± 1%, t = 0.129, P=0.552)均无明显变化(图3B、D)。
甲醛所致炎性痛模型是一种公认的无菌性炎症的理想模型, 此模型可模拟人类损伤后疼痛的某些特征, 且具有良好的重复性, 因此广泛应用于疼痛机制的研究[20]。与既往的研究结果一致[21], 我们的实验结果表明模型组大鼠注射足均表现出典型的二相痛反应。目前研究证实第一相急性伤害性反应是甲醛直接刺激伤害性感受器所致, 主要引起C类纤维的兴奋, 是短暂的急性痛反应, 由此触发了第二相的中枢敏感化, 因此第二相继发性的伤害性反应依赖于外周的炎症发展及中枢神经系统的参与。我们的研究结果进一步表明, 腹腔注射米诺环素不影响第一相, 但能显著抑制第二相, 表明腹腔注射米诺环素对急性炎性痛具有镇痛效应。c-Fos是即刻早期基因的蛋白产物, 通常被作为伤害性神经元激活的标志物, 诱导脊髓背角表达增加的相关机制目前尚未完全阐明, 早年的研究认为可能与异常神经冲动直接传入或者下行抑制通路神经的传入增加有关[22], 近年的研究发现脊髓c-Fos的增加与细胞内代谢型谷氨酸受体5亚型的激活有关[23]。此外, 多项研究表明γ -氨基丁酸(γ -aminobutyrate, GABA)释放增加可抑制脊髓背角c-Fos的表达[24]。本研究中, 米诺环素腹腔注射可抑制脊髓背角c-Fos的表达, 而米诺环素灌流3 min即可显著增加脊髓背角sIPSCs的频率, 即增加突触前GABA的释放, 这进一步表明米诺环素抑制c-Fos的表达与其增加脊髓背角GABA释放有关。
SG区即脊髓背角Ⅱ 层, 被认为是伤害性信息向中枢传递的重要门户。SG神经元不仅接收外周初级传入神经(主要为Aδ 纤维和C纤维)介导的伤害性信息, 还接收脊髓背角中间神经元和下行抑制性投射神经纤维的伤害性信息。脊髓的“ 去抑制” 被认为是导致神经病理性痛的重要机制之一[25, 26], 例如鞘内注射抑制性神经递质γ -氨基丁酸可缓解神经病理性痛[27, 28]。在本研究中, 我们发现米诺环素对SG神经元的兴奋性突触传递无明显影响, 但可显著增强SG神经元的抑制性突触传递, 这对进一步从细胞层面揭示米诺环素的镇痛作用具有重要的意义。
尽管米诺环素的镇痛效应已在多种疼痛模型上得以证明, 但其镇痛机制尚不明确。目前关于米诺环素的镇痛机制主要有以下几种观点:(1)米诺环素可抑制小胶质细胞活化, 通过减少炎性介质的生成释放而有效缓解疼痛[29]; (2)米诺环素通过影响谷氨酸受体和离子通道的功能, 降低神经元的兴奋性, 从而发挥其镇痛效应[30]; (3)米诺环素通过抑制细胞内激酶的磷酸化, 抑制细胞内信号通路等机制减轻疼痛[31]。
有研究表明米诺环素对海马神经元和SG神经元的兴奋性突触传递无明显影响[32, 33], 与我们观察到的实验结果一致。而Gonzá lez等[34]报道米诺环素可抑制海马神经元的兴奋性突触传递, 与本实验结果比较, 其差异性可能与所研究的神经元及局部神经回路不同有关。此外, 我们的研究揭示了米诺环素对SG神经元抑制性突触传递的作用。米诺环素可增加SG神经元的sIPSCs的频率而不影响其幅度, 提示米诺环素主要作用于突触前膜, 增加抑制性神经递质的释放, 而非作用于突触后膜, 未改变突触后膜递质受体的功能。然而米诺环素作为一种半合成四环素, 除用作抗生素外, 也常用作胶质细胞活化的抑制剂。氟代柠檬酸是另一种胶质细胞抑制剂, 尽管其鞘内注射能通过降低IL-6的产生从而对神经病理性痛大鼠产生镇痛效应[35], 但在本研究中, 氟代柠檬酸对SG神经元sIPSCs的频率和幅度均无明显影响。疼痛模型后胶质细胞的激活通常需数天至数周[36], 结合本研究中米诺环素可迅速(约3 min内)增强抑制性突触传递的作用, 提示米诺环素的镇痛效应与其对胶质细胞的抑制作用无关。此外, 灌流多西环素后, sIPSCs的频率和幅度无明显变化, 提示米诺环素的镇痛效应也与其抗生素特性无关。
本研究结果表明米诺环素可抑制甲醛所致炎性痛及脊髓背角c-Fos蛋白的表达, 且揭示了米诺环素可显著增强脊髓SG神经元的抑制性突触传递, 为其用于临床镇痛奠定了新的理论基础。
(本文编辑:刘淑萍)
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|
| [21] |
|
| [22] |
|
| [23] |
|
| [24] |
|
| [25] |
|
| [26] |
|
| [27] |
|
| [28] |
|
| [29] |
|
| [30] |
|
| [31] |
|
| [32] |
|
| [33] |
|
| [34] |
|
| [35] |
|
| [36] |
|