Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (6): 1150-1154. doi: 10.19723/j.issn.1671-167X.2019.06.030
Previous Articles Next Articles
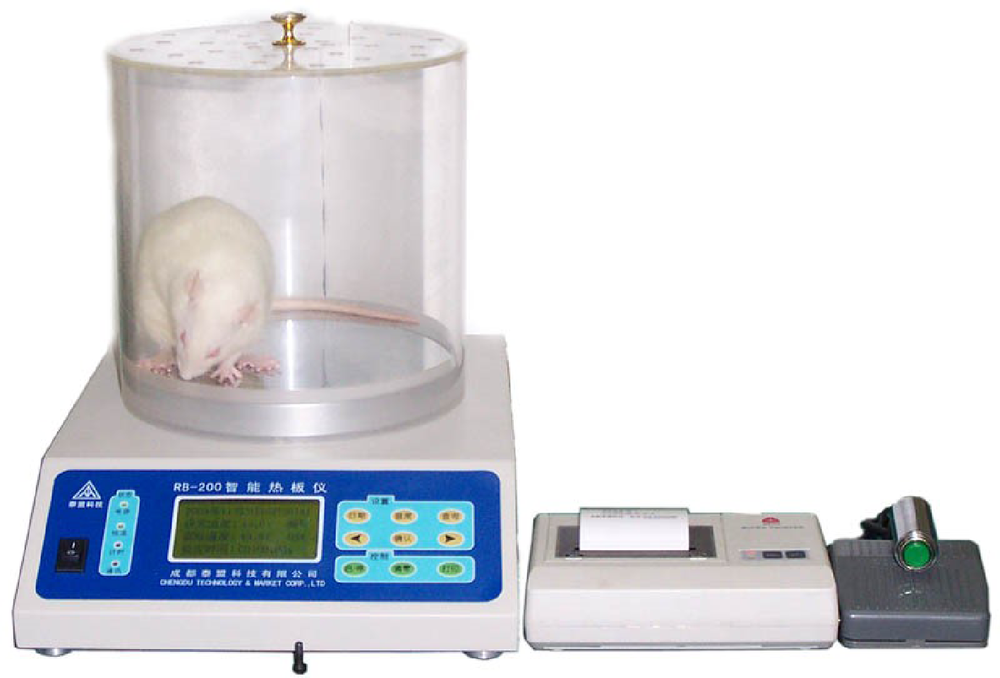
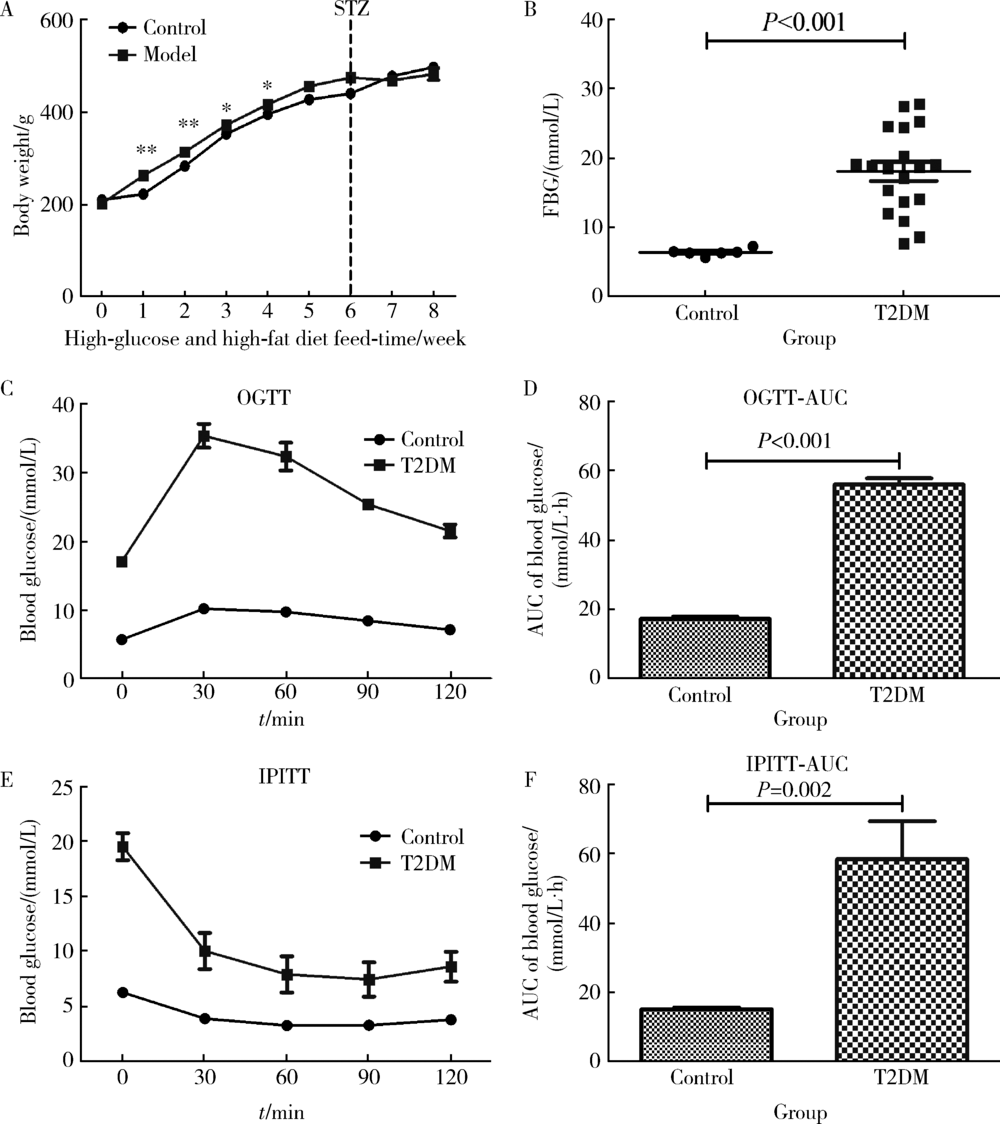
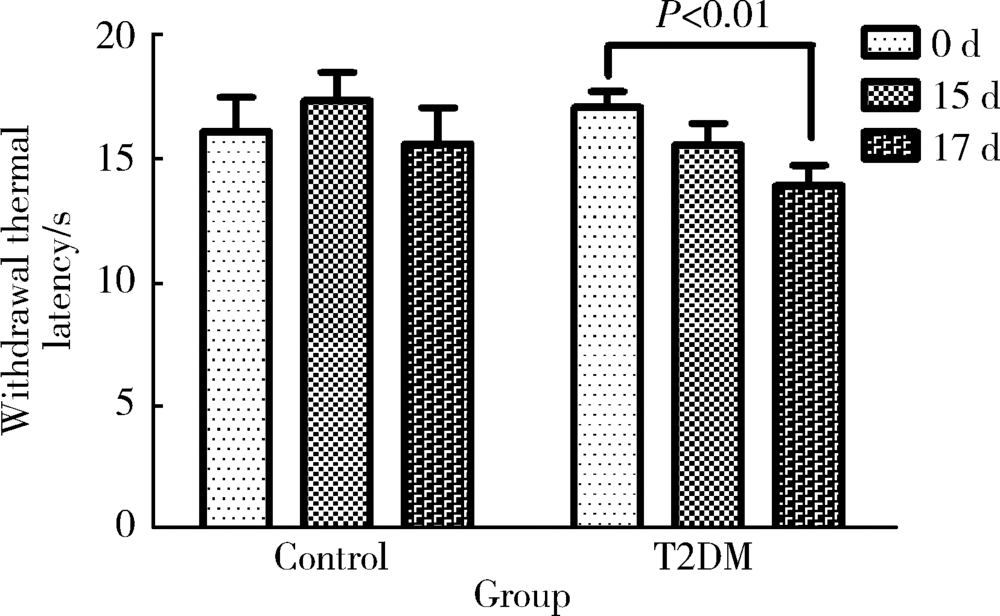
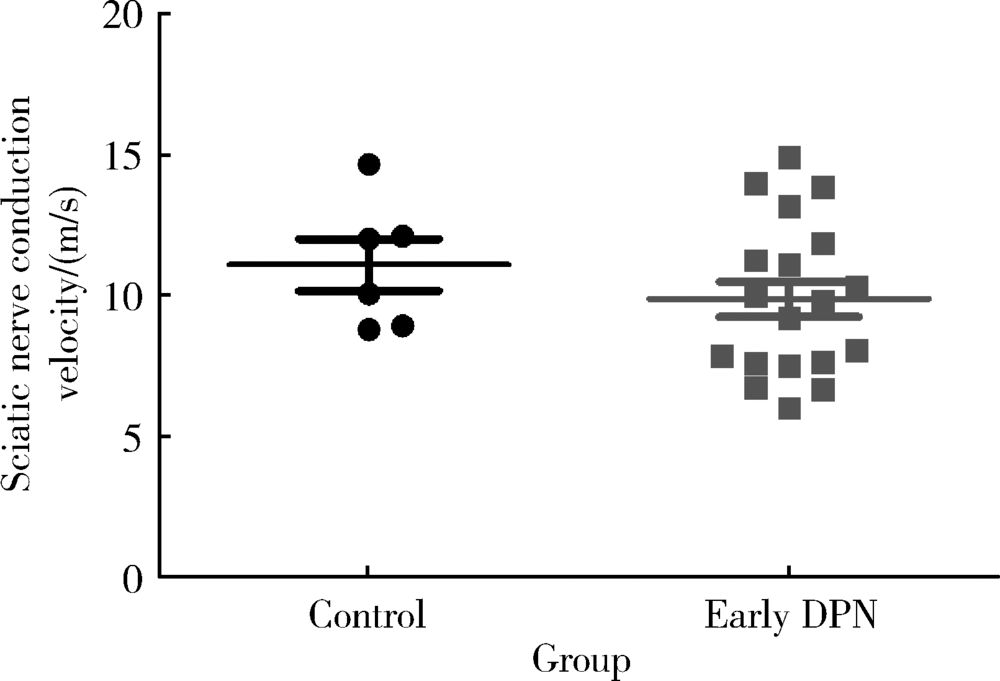
Approach to creating early diabetic peripheral neuropathy rat model
Jiao HE,Ge-heng YUAN( ),Jun-qing ZHANG,Xiao-hui GUO
),Jun-qing ZHANG,Xiao-hui GUO
- Department of Endocrinology, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R745
| [1] | Singh R, Kishore L, Kaur N . Diabetic peripheral neuropathy: current perspective and future directions[J]. Pharmacol Res, 2014,80:21-35. |
| [2] | Shi X, Chen Y, Nadeem L , et al. Beneficial effect of TNF-α inhibition on diabetic peripheral neuropathy[J]. J Neuroinflammation, 2013,10:69. |
| [3] | Bai J, Zhu Y, Dong Y . Response of gut microbiota and inflammatory status to bitter melon (Momordica charantia L.) in high fat diet induced obese rats[J]. J Ethnopharmacol, 2016,194:717-726. |
| [4] | Ishibashi K, Hara A, Fujitani Y , et al. Beneficial effects of vildagliptin combined with miglitol on glucose tolerance and islet morphology in diet-controlled db/db mice[J]. Biochem Biophys Res Commun, 2013,440(4):570-575. |
| [5] | Jolivalt CG, Frizzi KE, Guernsey L , et al. Peripheral neuropathy in mouse models of diabetes[J]. Curr Protoc Mouse Biol, 2016,6(3):223-255. |
| [6] | Balter RE, Dykstra LA . Thermal sensitivity as a measure of spontaneous morphine withdrawal in mice[J]. J Pharmacol Toxicol Methods, 2013,67(3):162-168. |
| [7] | Shi W, Ding Y, Yu A , et al. BDNF/TRK/KCC2 pathway in nicotine withdrawal-induced hyperalgesia[J]. Transl Neurosci, 2015,6(1):208-213. |
| [8] | 宋庆芳 . 游离脂肪酸与2型糖尿病周围神经病变的关系及其机制探讨[D]. 石家庄: 河北医科大学, 2010. |
| [9] | 吴庆秋 . 枸杞多糖对氧化应激诱导2型糖尿病大鼠周围神经细胞凋亡的保护作用及其机制研究[D]. 银川: 宁夏医科大学, 2010. |
| [10] | Ding Y, Dai X, Jiang Y , et al. Functional and morphological effects of grape seed proanthocyanidins on peripheral neuropathy in rats with type 2 diabetes mellitus[J]. Phytother Res, 2014,28(7):1082-1087. |
| [11] | Reed MJ, Meszaros K, Entes LJ , et al. A new rat model of type 2 diabetes: the fat-fed, streptozotocin-treated rat[J]. Metabolism, 2000,49(11):1390-1394. |
| [12] | Zhou JY, Zhou SW . Protection of trigonelline on experimental diabetic peripheral neuropathy[J]. Evid Based Complement Alternat Med, 2012,164219. doi: 10.1155/2012/164219. |
| [13] | 邢国平 . 糖尿病患者周围神经功能的神经电生理比较研究[D]. 天津: 天津医科大学, 2009. |
| [14] | Chéliout-Héraut F, Zrek N, Khemliche H , et al. Exploration of small fibers for testing diabetic neuropathies[J]. Joint Bone Spine, 2005,72(5):412-415. |
| [15] | Krämer HH, Rolke R, Bickel A , et al. Thermal thresholds predict painfulness of diabetic neuropathies[J]. Diabetes Care, 2004,27(10):2386-2391. |
| [16] | Hao GM, Liu YG, Wu Y , et al. The protective effect of the active components of ERPC on diabetic peripheral neuropathy in rats[J]. J Ethnopharmacol, 2017,202:162-171. |
| [17] | Xu X, Yang X, Zhang P , et al. Effects of exogenous galanin on neuropathic pain state and change of galanin and its receptors in DRG and SDH after sciatic nerve-pinch injury in rat[J]. PLoS One, 2012,7(5):e37621. |
| [18] | Liao C, Yang M, Zhong W , et al. Association of myelinated primary afferents impairment with mechanical allodynia in diabetic peripheral neuropathy: an experimental study in rats[J]. Oncotarget, 2017,8(38):64157-64169. |
| [1] | Chen YU,Chun LI,Yang-yi FAN,Yan XU. Co-existence of Guillain-Barré syndrome and Behcet syndrome: A case report [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 1146-1149. |
| [2] | Jian-hua ZHU,Jing WANG,Xiao-jing LIU,Chuan-bin GUO. Accuracy analysis of robotic assistant needle placement for trigeminal gasserian ganglion [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 973-976. |
| [3] | Shuai XU,Yang-shuo WANG,Shu LI,Hai-ying LIU. Guillain-Barre syndrome complicated on post-operation with renal carcinoma and meningioma: a case report [J]. Journal of Peking University(Health Sciences), 2019, 51(4): 775-777. |
| [4] | HU Ping, LUO Ying-ying, WU Jing, GAO Lei-li. Analysis of clinical features of 23 patients with POEMS syndrome [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 985-989. |