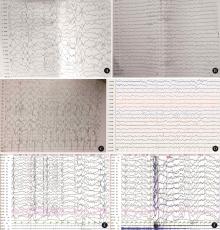
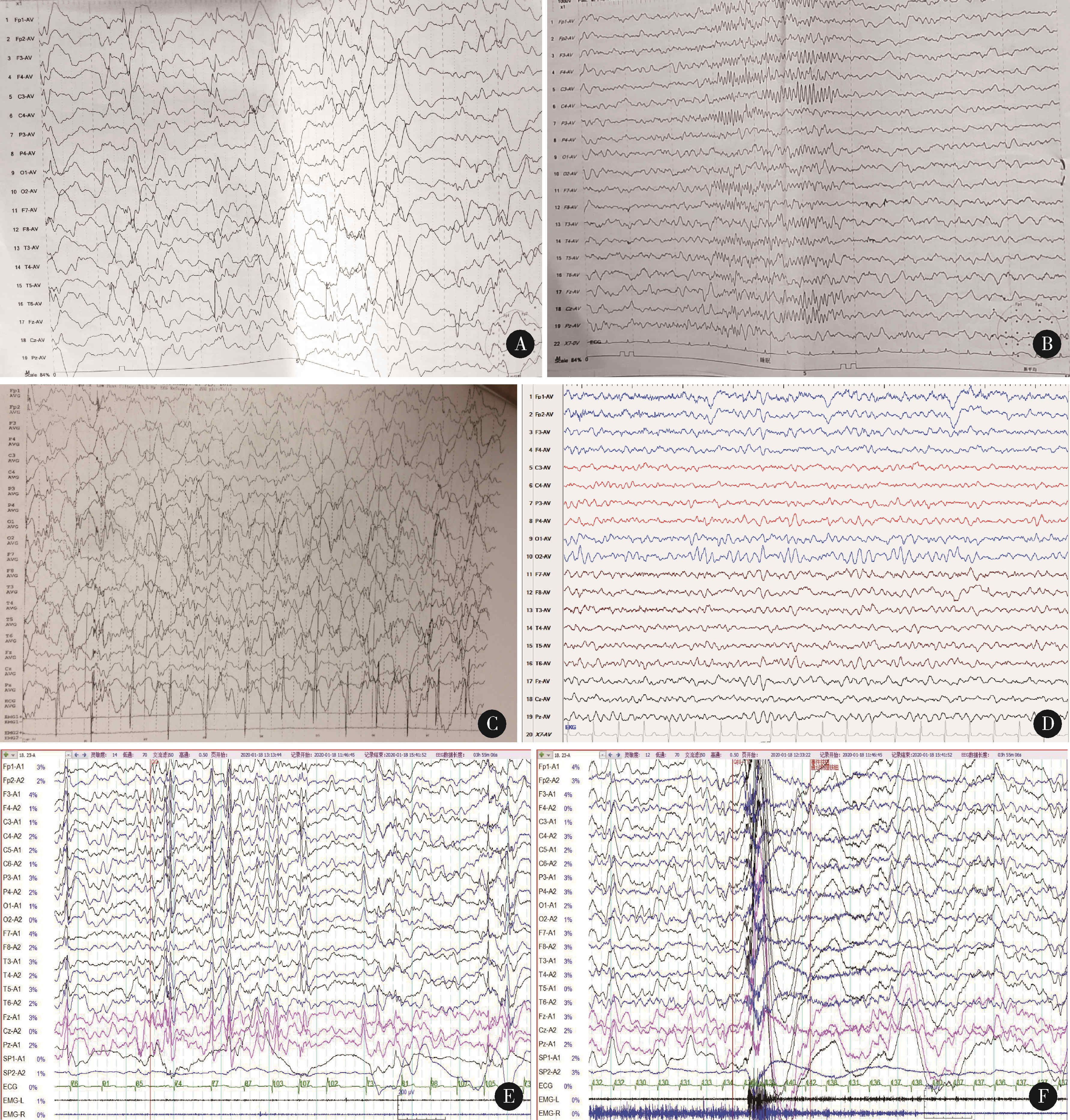
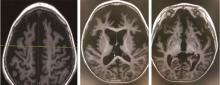
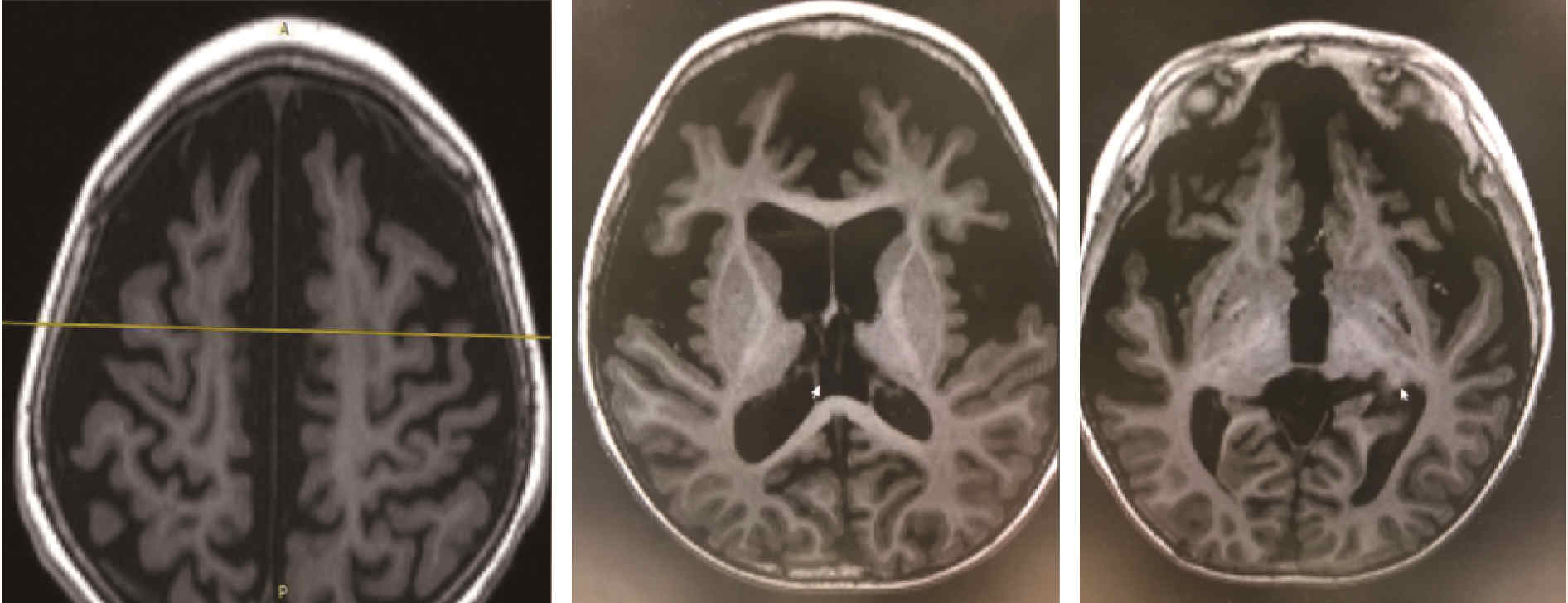
Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (5): 781-787. doi: 10.19723/j.issn.1671-167X.2024.05.005
Previous Articles Next Articles
Phenotype of infantile epileptic spasm syndrome in pyridoxin-dependent epilepsy
Xianru JIAO1,2, Pan GONG2, Yue NIU1, Zhao XU1, Zongpu ZHOU1, Zhixian YANG1,*( )
)
- 1. Department of Pediatrics, Peking University People's Hospital, Beijing 100044, China
2. Department of Pediatrics, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R742.1
| 1 |
Hunt AD Jr , Stokes J Jr , McCrory WW , et al. Pyridoxine dependency: Report of a case of intractable convulsions in an infant controlled by pyridoxine[J]. Pediatrics, 1954, 13 (2): 140- 145.
doi: 10.1542/peds.13.2.140 |
| 2 |
Mills PB , Struys E , Jakobs C . Mutations in antiquitin in individuals with pyridoxine-dependent seizures[J]. Nat Med, 2006, 12 (3): 307- 309.
doi: 10.1038/nm1366 |
| 3 |
Zuberi SM , Wirrell E , Yozawitz E , et al. ILAE classification and definition of epilepsy syndromes with onset in neonates and infants: Position statement by the ILAE task force on nosology and definitions[J]. Epilepsia, 2022, 63 (6): 1349- 1397.
doi: 10.1111/epi.17239 |
| 4 |
Jiao X , Xue J , Gong P , et al. Clinical and genetic features in pyridoxine-dependent epilepsy: A Chinese cohort study[J]. Dev Med Child Neurol, 2020, 62 (3): 315- 321.
doi: 10.1111/dmcn.14385 |
| 5 |
Tseng LA , Abdenur JE , Andrews A , et al. Timing of therapy and neurodevelopmental outcomes in 18 families with pyridoxine-dependent epilepsy[J]. Mol Genet Metab, 2022, 135 (4): 350- 356.
doi: 10.1016/j.ymgme.2022.02.005 |
| 6 |
Strijker M , Tseng LA , van Avezaath LK , et al. Cognitive and neurological outcome of patients in the Dutch pyridoxine-dependent epilepsy (PDE-ALDH7A1) cohort, a cross-sectional study[J]. Eur J Paediatr Neurol, 2021, 33, 112- 120.
doi: 10.1016/j.ejpn.2021.06.001 |
| 7 |
Struys EA , Bok LA , Emal D , et al. The measurement of urinary Delta(1)-piperideine-6-carboxylate, the alter ego of alpha-aminoadipic semialdehyde, in Antiquitin deficiency[J]. J Inherit Metab Dis, 2012, 35 (5): 909- 916.
doi: 10.1007/s10545-011-9443-0 |
| 8 |
Struys EA , Jakobs C . Metabolism of lysine in alpha-aminoadipic semialdehyde dehydrogenase-deficient fibroblasts: Evidence for an alternative pathway of pipecolic acid formation[J]. FEBS Lett, 2010, 584 (1): 181- 186.
doi: 10.1016/j.febslet.2009.11.055 |
| 9 |
Jansen LA , Hevner RF , Roden WH , et al. Glial localization of antiquitin: Implications for pyridoxine-dependent epilepsy[J]. Ann Neurol, 2014, 75 (1): 22- 32.
doi: 10.1002/ana.24027 |
| 10 |
Lux AL , Osborne JP . A proposal for case definitions and outcome measures in studies of infantile spasms and West syndrome: Consensus statement of the West Delphi group[J]. Epilepsia, 2004, 45 (11): 1416- 1428.
doi: 10.1111/j.0013-9580.2004.02404.x |
| 11 |
van Karnebeek CD , Tiebout SA , Niermeijer J , et al. Pyridoxine-dependent epilepsy: An expanding clinical spectrum[J]. Pediatr Neurol, 2016, 59, 6- 12.
doi: 10.1016/j.pediatrneurol.2015.12.013 |
| 12 |
Basura GJ , Hagland SP , Wiltse AM , et al. Clinical features and the management of pyridoxine-dependent and pyridoxine-responsive seizures: Review of 63 North American cases submitted to a patient registry[J]. Eur J Pediatr, 2009, 168 (6): 697- 704.
doi: 10.1007/s00431-008-0823-x |
| 13 |
Mefford HC , Zemel M , Geraghty E , et al. Intragenic deletions of ALDH7A1 in pyridoxine-dependent epilepsy caused by Alu-Alu recombination[J]. Neurology, 2015, 85 (9): 756- 762.
doi: 10.1212/WNL.0000000000001883 |
| 14 |
Pérez B , Gutiérrez-Solana LG , Verdú A , et al. Clinical, biochemical, and molecular studies in pyridoxine-dependent epilepsy. Antisense therapy as possible new therapeutic option[J]. Epilepsia, 2013, 54 (2): 239- 248.
doi: 10.1111/epi.12083 |
| 15 |
Bennett CL , Chen Y , Hahn S , et al. Prevalence of ALDH7A1 mutations in 18 North American pyridoxine-dependent seizure (PDS) patients[J]. Epilepsia, 2009, 50 (5): 1167- 1175.
doi: 10.1111/j.1528-1167.2008.01816.x |
| 16 |
Al Teneiji A , Bruun TU , Cordeiro D , et al. Phenotype, biochemical features, genotype, and treatment outcome of pyridoxine-dependent epilepsy[J]. Metab Brain Dis, 2017, 32 (2): 443- 451.
doi: 10.1007/s11011-016-9933-8 |
| 17 |
Falsaperla R , Vari MS , Toldo I , et al. Pyridoxine-dependent epilepsies: An observational study on clinical, diagnostic, therapeutic and prognostic features in a pediatric cohort[J]. Metab Brain Dis, 2018, 33 (1): 261- 269.
doi: 10.1007/s11011-017-0150-x |
| 18 |
Scharer G , Brocker C , Vasiliou V , et al. The genotypic and phenotypic spectrum of pyridoxine-dependent epilepsy due to mutations in ALDH7A1[J]. J Inherit Metab Dis, 2010, 33 (5): 571- 581.
doi: 10.1007/s10545-010-9187-2 |
| 19 |
Tlili A , Hamida Hentati N , Chaabane R , et al. Pyridoxine-dependent epilepsy in Tunisia is caused by a founder missense mutation of the ALDH7A1 gene[J]. Gene, 2013, 518 (2): 242- 245.
doi: 10.1016/j.gene.2013.01.041 |
| 20 | Mills PB , Footitt EJ , Mills KA , et al. Genotypic and phenotypic spectrum of pyridoxine-dependent epilepsy (ALDH7A1 deficiency)[J]. Brain, 2010, 133 (Pt 7): 2148- 2159. |
| 21 |
Gibaud M , Barth M , Lefranc J , et al. West syndrome is an exceptional presentation of pyridoxine- and pyridoxal phosphate-dependent epilepsy: Data from a French cohort and review of the literature[J]. Front Pediatr, 2021, 9, 621200.
doi: 10.3389/fped.2021.621200 |
| 22 | Mastrangelo M , Gasparri V , Bernardi K , et al. Epilepsy phenotypes of vitamin B6-dependent diseases: An updated systematic review[J]. Children (Basel), 2023, 10 (3): 553. |
| 23 |
Srinivasaraghavan R , Parameswaran N , Mathis D , et al. Antiquitin deficiency with adolescent onset epilepsy: Molecular diagnosis in a mother of affected offsprings[J]. Neuropediatrics, 2018, 49 (2): 154- 157.
doi: 10.1055/s-0037-1621721 |
| 24 |
Baxter P . Epidemiology of pyridoxine dependent and pyridoxine responsive seizures in the UK[J]. Arch Dis Child, 1999, 81, 431- 433.
doi: 10.1136/adc.81.5.431 |
| 25 | Yeghiazaryan NS , Zara F , Capovilla G , et al. Pyridoxine-dependent epilepsy: An under-recognised cause of intractable seizures[J]. J Paediatr Child Health, 2012, 48 (3): E113- E115. |
| 26 |
Haidar Z , Jalkh N , Corbani S , et al. Atypical pyridoxine dependent epilepsy resulting from a new homozygous missense mutation, in ALDH7A1[J]. Seizure, 2018, 57, 32- 33.
doi: 10.1016/j.seizure.2018.03.010 |
| 27 |
Marguet F , Barakizou H , Tebani A , et al. Pyridoxine-dependent epilepsy: Report on three families with neuropathology[J]. Metab Brain Dis, 2016, 31 (6): 1435- 1443.
doi: 10.1007/s11011-016-9869-z |
| 28 |
Coughlin CR , Tseng LA , Abdenur JE , et al. Consensus guidelines for the diagnosis and management of pyridoxine-dependent epilepsy due to alpha-aminoadipic semialdehyde dehydrogenase deficiency[J]. J Inherit Metab Dis, 2021, 44 (1): 178- 192.
doi: 10.1002/jimd.12332 |
| 29 | 杨志仙, 秦炯. 吡哆醇依赖性癫痫的临床及分子遗传学研究进展[J]. 中华儿科杂志, 2013, 51 (11): 867- 870. |
| 30 | van Karnebeek CD , Jaggumantri S . Current treatment and management of pyridoxine-dependent epilepsy[J]. Curr Treat Options Neurol, 2015, 17 (2): 335. |
| 31 | Ohtsuka Y , Yamatogi Y , Yoshida H , et al. High-dose pyridoxal phosphate in the treatment of the West and the Lennox syndromes[J]. No To Hattatsu, 1983, 15 (3): 225- 233. |
| 32 |
Ohtsuka Y , Matsuda M , Ogino T , et al. Treatment of the West syndrome with high-dose pyridoxal phosphate[J]. Brain Dev, 1987, 9 (4): 418- 421.
doi: 10.1016/S0387-7604(87)80116-X |
| 33 |
Mytinger JR , Joshi S . The current evaluation and treatment of infantile spasms among members of the child neurology society[J]. J Child Neurol, 2012, 27 (10): 1289- 1294.
doi: 10.1177/0883073812455692 |
| 34 |
Pietz J , Benninger C , Schäfer H , et al. Treatment of infantile spasms with high-dosage vitamin B6[J]. Epilepsia, 1993, 34 (4): 757- 763.
doi: 10.1111/j.1528-1157.1993.tb00458.x |
| 35 |
Xue J , Qian P , Li H , et al. Clinical characteristics of two cohorts of infantile spasms: Response to pyridoxine or topiramate monotherapy[J]. World J Pediatr, 2018, 14 (3): 290- 297.
doi: 10.1007/s12519-018-0127-9 |
| [1] | Sha ZHU,Zong-sheng XU,Qing XIA,Xiao-jing FANG,Dan-hua ZHAO,Xian-zeng LIU. Clinico-pathological features of temporal lobe epilepsy with enlarged amygdala [J]. Journal of Peking University(Health Sciences), 2019, 51(5): 824-828. |
| [2] | GONG Pan, YANG Zhi-xian, XUE Jiao, QIAN Ping, YANG Hai-po, LIU Xiao-yan,BIAN Kai-gui. Application of scalp-recorded high-frequency oscillations in epileptic encephalopathy with continuous spike-and-wave during sleep [J]. Journal of Peking University(Health Sciences), 2018, 50(2): 213-220. |
|
||