Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (2): 234-242. doi: 10.19723/j.issn.1671-167X.2023.02.006
Previous Articles Next Articles
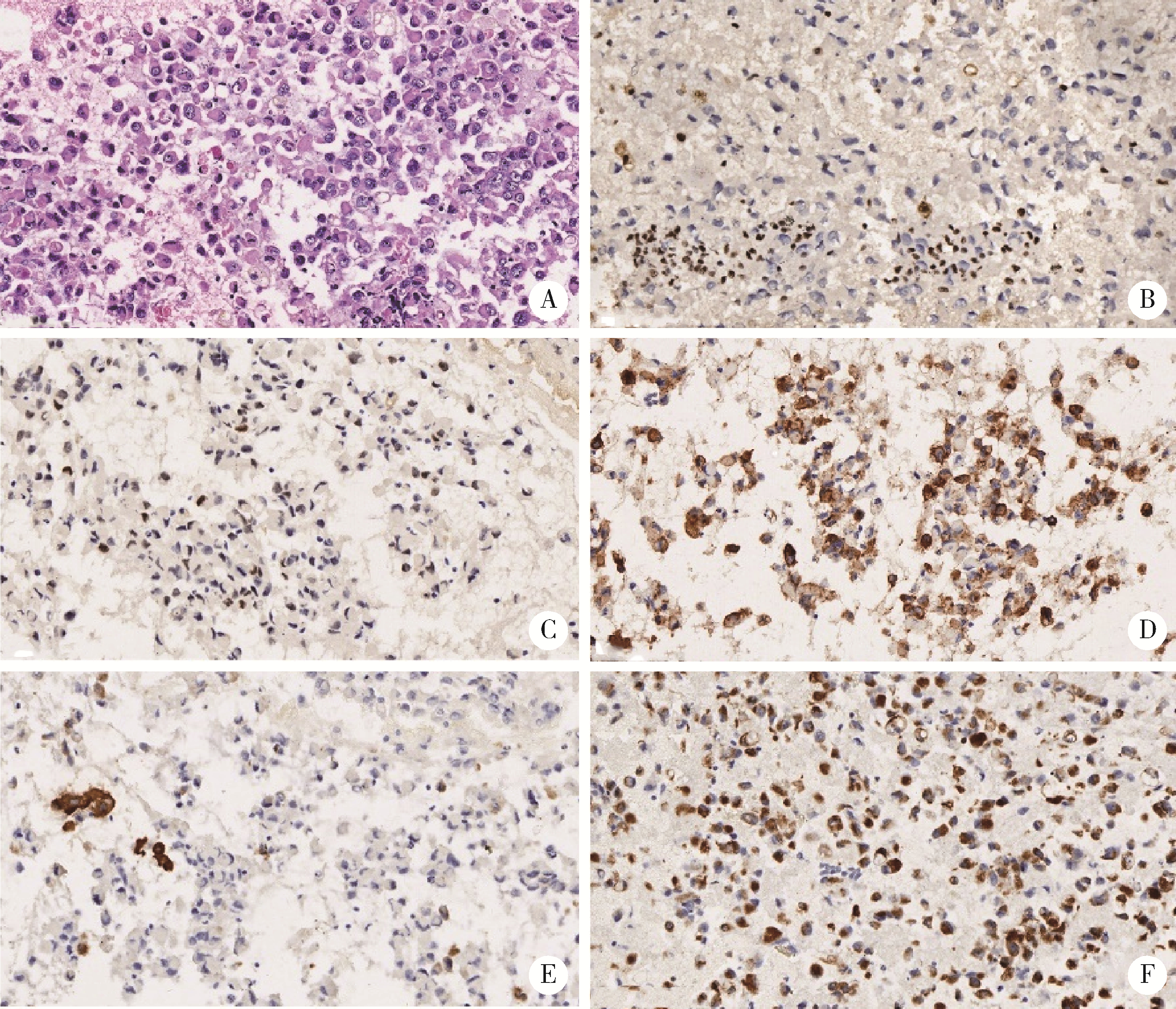
Evaluation of accuracy of pathological diagnosis based on thyroid core needle biopsy
Yan XIONG1,*( ),Xin LI1,Li LIANG1,Dong LI1,Li-min YAN1,Xue-ying LI2,Ji-ting DI1,Ting LI1
),Xin LI1,Li LIANG1,Dong LI1,Li-min YAN1,Xue-ying LI2,Ji-ting DI1,Ting LI1
- 1. Department of Pathology, Peking University First Hospital, Beijing 100034, China
2. Department of Biostatistics, Peking University First Hospital, Beijing 100034, China
CLC Number:
- R736.1
| 1 | Teng WLY , Gao M , Huang G , et al. Guidelines on the diagnosis and treatment of thyroid nodules and differentiated thyroid carcinomas[J]. Chin J Endocrinol Metab, 2012, 28 (10): 779- 797. |
| 2 |
Pitman MB , Abele J , Ali SZ , et al. Techniques for thyroid FNA: A synopsis of the National Cancer Institute Thyroid Fine-Needle Aspiration State of the Science Conference[J]. Diagn Cytopathol, 2008, 36 (6): 407- 424.
doi: 10.1002/dc.20829 |
| 3 |
Suster S . Controversies regarding the interpretation of follicular thyroid nodules[J]. Arch Pathol Lab Med, 2019, 143 (12): 1472- 1476.
doi: 10.5858/arpa.2019-0301-RA |
| 4 |
Ha EJ , Baek JH , Lee JH , et al. Complications following US-guided core-needle biopsy for thyroid lesions: A retrospective study of 6 169 consecutive patients with 6 687 thyroid nodules[J]. Eur Radiol, 2017, 27 (3): 1186- 1194.
doi: 10.1007/s00330-016-4461-9 |
| 5 |
Jung CK , Baek JH , Na DG , et al. 2019 Practice guidelines for thyroid core needle biopsy: A report of the Clinical Practice Guidelines Development Committee of the Korean Thyroid Association[J]. J Pathol Transl Med, 2020, 54 (1): 64- 86.
doi: 10.4132/jptm.2019.12.04 |
| 6 | Lloyd RV , Osamura RY , Klöppel G . WHO classification of tumours of endocrine organs[M]. 4th ed Lyon: International Agency for Research on Cancer (IARC), 2017: 65- 142. |
| 7 |
Dunderovic D , Lipkovski JM , Boricic I , et al. Defining the value of CD56, CK19, Galectin 3 and HBME-1 in diagnosis of follicular cell derived lesions of thyroid with systematic review of literature[J]. Diagn Pathol, 2015, 10, 196.
doi: 10.1186/s13000-015-0428-4 |
| 8 | Gharib H , Papini E , Garber JR , et al. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update[J]. Endocr Pract, 2016, 22 (5): 622- 639. |
| 9 |
Haugen BR , Alexander EK , Bible KC , et al. 2015 American Thyroid Association Management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: The American Thyroid Association Guidelines Task Force on thyroid nodules and differentiated thyroid cancer[J]. Thyroid, 2016, 26 (1): 1- 133.
doi: 10.1089/thy.2015.0020 |
| 10 |
Na DG , Baek JH , Jung SL , et al. Core needle biopsy of the thyroid: 2016 Consensus Statement and Recommendations from Korean Society of Thyroid Radiology[J]. Korean J Radiol, 2017, 18 (1): 217- 237.
doi: 10.3348/kjr.2017.18.1.217 |
| 11 | Travis WDBE , Burke AP , Marx A , et al. WHO classification of tumurs of the lung, pleura, thymus and hear[M]. 4th ed Lyon: International Agency for Research on Cancer (IARC), 2015: 29- 36. |
| 12 |
Jung CK , Min HS , Park HJ , et al. Pathology reporting of thyroid core needle biopsy: A proposal of the Korean Endocrine Pathology Thyroid Core Needle Biopsy Study Group[J]. J Pathol Transl Med, 2015, 49 (4): 288- 299.
doi: 10.4132/jptm.2015.06.04 |
| 13 |
Wolinski K , Stangierski A , Ruchala M . Comparison of diagnostic yield of core-needle and fine-needle aspiration biopsies of thyroid lesions: Systematic review and meta-analysis[J]. Eur Radiol, 2017, 27 (1): 431- 436.
doi: 10.1007/s00330-016-4356-9 |
| 14 |
Song S , Kim H , Ahn SH . Role of immunohistochemistry in fine needle aspiration and core needle biopsy of thyroid nodules[J]. Clin Exp Otorhinolaryngol, 2019, 12 (2): 224- 230.
doi: 10.21053/ceo.2018.01011 |
| 15 |
Cancer Genome Atlas Research Network . Integrated genomic characterization of papillary thyroid carcinoma[J]. Cell, 2014, 159 (3): 676- 690.
doi: 10.1016/j.cell.2014.09.050 |
| 16 |
D'Cruz AK , Vaish R , Vaidya A , et al. Molecular markers in well-differentiated thyroid cancer[J]. Eur Arch Otorhinolaryngol, 2018, 275 (6): 1375- 1384.
doi: 10.1007/s00405-018-4944-1 |
| 17 |
Landa I , Ibrahimpasic T , Boucai L , et al. Genomic and transcriptomic hallmarks of poorly differentiated and anaplastic thyroid cancers[J]. J Clin Invest, 2016, 126 (3): 1052- 1066.
doi: 10.1172/JCI85271 |
| 18 |
Patel KN , Angell TE , Babiarz J , et al. Performance of a genomic sequencing classifier for the preoperative diagnosis of cytologically indeterminate thyroid nodules[J]. JAMA Surg, 2018, 153 (9): 817- 824.
doi: 10.1001/jamasurg.2018.1153 |
| 19 | Sadowski SM , Petrenko V , Meyer P , et al. Validation of molecular biomarkers for preoperative diagnostics of human papillary thyroid carcinoma in fine needle aspirates[J]. Gland Surg, 2019, 8 (Suppl 2): S62- S76. |
| 20 |
Choi SH , Baek JH , Lee JH , et al. Evaluation of the clinical usefulness of BRAFV600E mutation analysis of core-needle biopsy specimens in thyroid nodules with previous atypia of undetermined significance or follicular lesions of undetermined significance results[J]. Thyroid, 2015, 25 (8): 897- 903.
doi: 10.1089/thy.2014.0606 |
| 21 |
Choi SH , Baek JH , Lee JH , et al. Initial clinical experience with BRAF(V600E) mutation analysis of core-needle biopsy specimens from thyroid nodules[J]. Clin Endocrinol, 2016, 84 (4): 607- 613.
doi: 10.1111/cen.12866 |
| 22 | Crescenzi A , Trimboli P , Modica DC , et al. Preoperative assessment of TERT promoter mutation on thyroid core needle biopsies supports diagnosis of malignancy and addresses surgical strategy[J]. Horm Metab Res, 2016, 48 (3): 157- 162. |
| 23 |
Jang EK , Kim WG , Kim EY , et al. Usefulness of NRAS codon 61 mutation analysis and core needle biopsy for the diagnosis of thyroid nodules previously diagnosed as atypia of undetermined significance[J]. Endocrine, 2016, 52 (2): 305- 312.
doi: 10.1007/s12020-015-0773-9 |
| 24 |
Marotta V , Bifulco M , Vitale M . Significance of RAS mutations in thyroid benign nodules and non-medullary thyroid cancer[J]. Cancers (Basel), 2021, 13 (15): 3785.
doi: 10.3390/cancers13153785 |
| [1] | Peng FU,Wen CHEN,Li-gang CUI,Hui-yu GE,Shu-min WANG. Applicational value of 2017 ACR TI-RADS stratification in diagnosing thyroid nodules [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1067-1070. |
|
||