Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (5): 824-828. doi: 10.19723/j.issn.1671-167X.2019.05.006
Previous Articles Next Articles
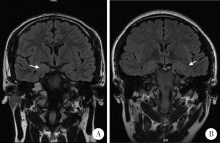
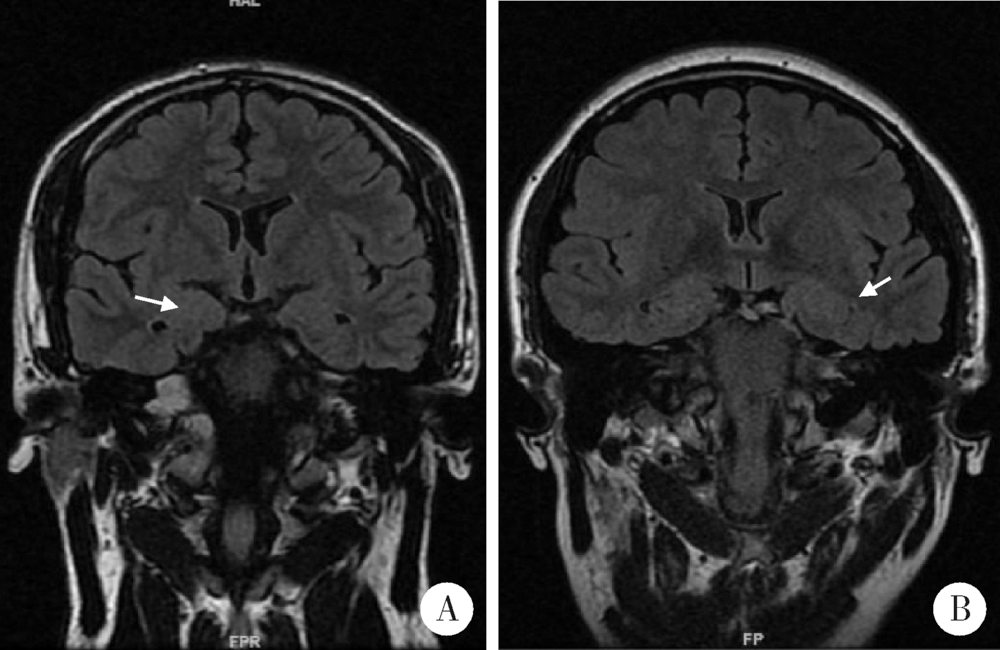
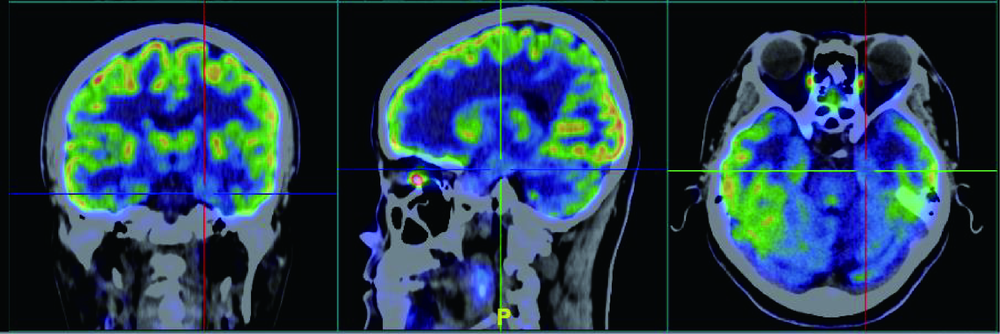
Clinico-pathological features of temporal lobe epilepsy with enlarged amygdala
Sha ZHU1,Zong-sheng XU1,Qing XIA1,Xiao-jing FANG1,Dan-hua ZHAO1,Xian-zeng LIU1,2,△( )
)
- 1. Department of Neurology, Peking University International Hospital, Beijing 102206, China
2. Department of Neurology, Peking University People’s Hospital, Beijing 100044, China
CLC Number:
- R742.1
| [1] | Soeder BM, Gleissner U, Urbach H , et al. Causes, presentation and outcome oflesional adult onset mediotemporal lobe epilepsy[J]. Neurol Neurosurg Psychiatry, 2009,80(8):894-899. |
| [2] | Goncalves Pereira PM, Oliveira E, Rosado P . Relative localizing value of amygdalohippocampal MR biometry in temporal lobe epilepsy[J]. Epilepsy Res, 2006,69(2):147-164. |
| [3] | Coan AC, Morita ME, de Campos BM, et al. Amygdala enlargement in patients with mesial temporal lobe epilepsy without hip-pocampal sclerosis[J]. Front Neurol, 2013,4:166. |
| [4] | Watson C, Andermann F, Gloor P , et al. Anatomic basis of amygdaloid and hippocampal volume measurement by magnetic resonance imaging[J]. Neurology, 1992,42(9):1743-1750. |
| [5] | Cendes F, Andermann F, Gloor P , et al. MRI volumetric mea-surement of amygdala and hippocampus intemporal lobe epilepsy[J]. Neurology, 1993,43(4):719-725. |
| [6] | Cendes F, Andermann F, Gloor P , et al. Relationship between atrophy of the amygdala and ictal fear in temporal lobe epilepsy[J]. Brain, 1994,117(4):739-746. |
| [7] | Bower SP, Vogrin SJ, Morris K , et al. Amygdala volumetry in “imaging-negative” temporal lobe epilepsy[J]. J Neurol Neurosurg Psychiatr, 2003,74(9):1245-1249. |
| [8] | Blumcke I, Thom M, Aronica E , et al. International consensus classification of hippocampal sclerosis in temporal lobe epilepsy: a task force report from the ILAE commission on diagnostic methods[J]. Epilepsia, 2013,54(7):1315-1329. |
| [9] | Beh SMJ, Cook MJ, D'Souza WJ . Isolated amygdala enlargement in temporal lobe epilepsy: A systematic review[J]. Epilepsy Behav, 2016,60:33-41. |
| [10] | 潘克梫, 陈楠, 王星 , 等. 1000名中国正常成人杏仁核体积高分辨MRI测量[J]. 中国医学影像技术, 2010,26(9):1615-1619. |
| [11] | Lv RJ, Sun ZR, Cui T , et al. Temporal lobe epilepsy with amygdala enlargement: a subtype of temporal lobe epilepsy[J]. BMC Neurol, 2014,14:194. |
| [12] | Tebartz van Elst L, Woermann FG, Lemieux L , et al. Amygdala enlargement in dysthymia—a volumetric study of patients with temporal lobe epilepsy[J]. Biol Psychiatry, 1999,46(12):1614-1623. |
| [13] | Tebartz van Elst L, Baeumer D, Lemieux L , et al. Amygdala pathology in psychosis of epilepsy: amagnetic resonance imaging study in patients with temporal lobe epilepsy[J]. Brain, 2002,125(1):140-149. |
| [14] | Morrell F, Wada J, Engel J . Potential relevance of kindling and secondary epileptogenesis to the consideration of surgical treatment for epilepsy[M] // Engel J. Surgical treatment of the epilepsies. New York: Raven, 1987: 701-707. |
| [15] | Gloor P, Olivier A, Quesney LF , et al. The role of the limbic system in experiential phenomena of temporal lobe epilepsy[J]. Ann Neurol, 1982,12(2):129-144. |
| [16] | Paesschen WV, Connelly A, Johnson CL , et al. The amygdala and intractable temporal lobe epilepsy: a quantitative magnetic resonance imaging study[J]. Neurology, 1996,47(4):1021-1031. |
| [17] | Wieser HG . Mesial temporal lobe epilepsy versus amygdalar epilepsy: late seizure recurrence after initially successful amygdalotomy and regained seizure control following hippocampectomy[J]. Epileptic Disord, 2000,2(3):141-152. |
| [18] | Bartolomei F, Wendling F, Regis J , et al. Pre-ictal synchronicity in limbic networks of mesial temporal lobe epilepsy[J]. Epilepsy Res, 2004,61(1-3):89-104. |
| [19] | Takaya S, Ikeda A, Mitsueda-Ono T , et al. Temporal lobe epilepsy with amygdala enlargement: a morphologic and functional study[J]. J Neuroimaging, 2014,24(1):54-62. |
| [20] | Minami N, Morino M, Uda T , et al. Surgery for amygdale enlargement with mesial temporal lobe epilepsy: pathological findings and seizure outcome[J]. J Neurol Neurosurg Psychiatry, 2015,86(8):887-894. |
| [21] | Bien CG, Urbach H, Schramm J , et al. Limbic encephalitis as a precipitating event in adult-onset temporal lobe epilepsy[J]. Neurology, 2007,69(12):1236-1244. |
| [22] | Kimura Y, Sato N, Saito Y , et al. Temporal lobe epilepsy with unilateral amygdala enlargement: morphometric MR analysis with clinical and pathological study[J]. J Neuroimaging, 2015,25(2):175-183. |
| [23] | Kim DW, Lee SK, Chung CK , et al. Clinical features and pathological characteristics of amygdala enlargement in mesial temporal lobe epilepsy[J]. J Clin Neurosci, 2012,19(4):509-512. |
| [24] | Phi JH, Kim SK, Cho BK , et al. Long-term surgical outcomes of temporal lobe epilepsy associated with low-grade brain tumors[J]. Cancer, 2009,115(24):5771-5779. |
| [25] | Eriksson SH, Nordborg C, Rydenhag B , et al. Parenchymal lesions in pharmacoresistant temporal lobe epilepsy: dual and multiple pathology[J]. Acta Neurol Scand, 2005,112(3):151-156. |
| [1] | Zhi-ming SUN,Qian CHEN,Ming-hua LI,Wei-ning MA,Xu-yang ZHAO,Zhuo HUANG. Chronic phosphoproteomic in temporal lobe epilepsy mouse models induced by kainic acid [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 197-205. |
|
||