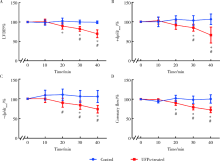
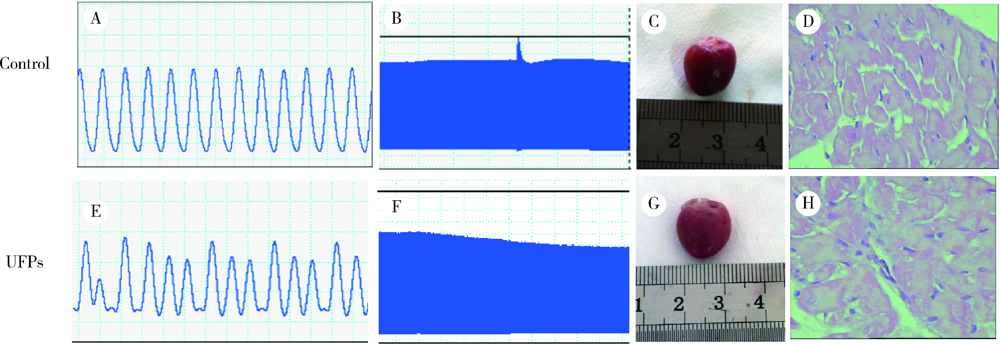
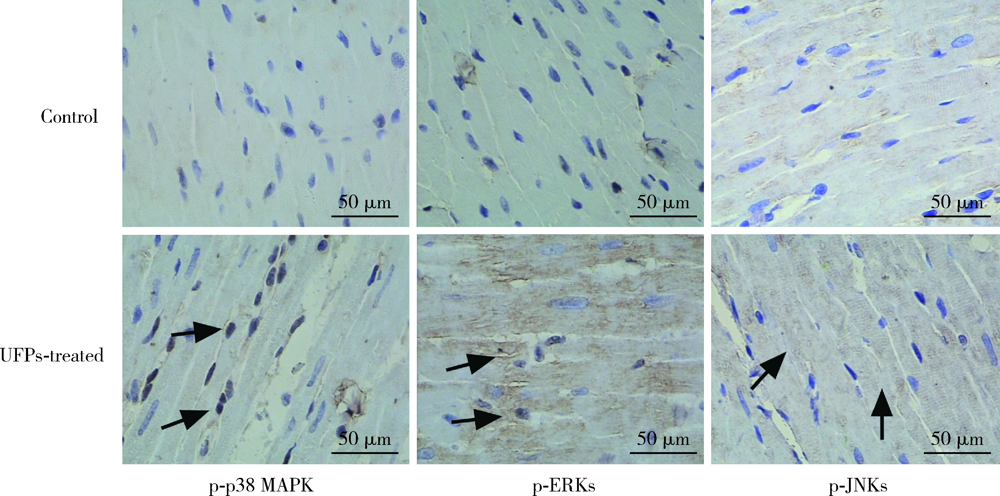
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (2): 240-245. doi: 10.19723/j.issn.1671-167X.2021.02.002
Previous Articles Next Articles
Effects of ultrafine particulates on cardiac function in rat isolated heart
BAI Feng,HE Yi-fan,NIU Ya-nan,YANG Ruo-juan,CAO Jing( )
)
- Department of Cardiology, The First Hospital of Shanxi Medical University & Department of Pharmacology, Basic Medical School, Shanxi Medical University, Taiyuan 030001, China
CLC Number:
- R122.7
| [1] | Klompmaker JO, Montagne DR, Meliefste K, et al. Spatial variation of ultrafine particles and black carbon in two cities: results from a short-term measurement campaign[J]. Sci Total Environ, 2015,508:266-275. |
| [2] |
Zareba W, Couderc JP, Oberdörster G, et al. ECG parameters and exposure to carbon ultrafine particles in young healthy subjects[J]. Inhal Toxicol, 2009,21(3):223-233.
doi: 10.1080/08958370802492407 pmid: 18991063 |
| [3] |
Laumbach RJ, Kipen HM, Ko S, et al. A controlled trial of acute effects of human exposure to traffic particles on pulmonary oxidative stress and heart rate variability[J]. Part Fibre Toxicol, 2014,11:45.
pmid: 25361615 |
| [4] |
Mills NL, Amin N, Robinson SD, et al. Do inhaled carbon nanoparticles translocate directly into the circulation in humans[J]. Am J Respir Crit Care Med, 2006,173(4):426-431.
pmid: 16339922 |
| [5] |
Lundbäck M, Mills NL, Lucking A, et al. Experimental exposure to diesel exhaust increases arterial stiffness in man[J]. Part Fibre Toxicol, 2009,6:7.
pmid: 19284640 |
| [6] |
Wang M, Beelen R, Stafoggia M, et al. Long-term exposure to elemental constituents of particulate matter and cardiovascular mortality in 19 European cohorts: results from the ESCAPE and TRANSPHORM projects[J]. Environ Int, 2014,66:97-106.
doi: 10.1016/j.envint.2014.01.026 pmid: 24561271 |
| [7] |
Stewart JC, Chalupa DC, Devlin RB, et al. Vascular effects of ultrafine particles in persons with type 2 diabetes[J]. Environ Health Perspect, 2010,118(12):1692-1698.
pmid: 20822968 |
| [8] |
Nemmar A, Subramaniyan D, Yasin J, et al. Impact of experimental type 1 diabetes mellitus on systemic and coagulation vulnerability in mice acutely exposed to diesel exhaust particles[J]. Part Fibre Toxicol, 2013,10:14.
pmid: 23587270 |
| [9] |
Rückerl R, Phipps RP, Schneider A, et al. Ultrafine particles and platelet activation in patients with coronary heart disease: results from a prospective panel study[J]. Part Fibre Toxicol, 2007,4:1.
doi: 10.1186/1743-8977-4-1 pmid: 17241467 |
| [10] |
Sun Q, Yue P, Ying Z, et al. Air pollution exposure potentiates hypertension through reactive oxygen species-mediated activation of Rho/ROCK[J]. Arterioscler Thromb Vasc Biol, 2008,28(10):1760-1766.
pmid: 18599801 |
| [11] | Simkhovich BZ, Marjoram P, Kleinman MT, et al. Direct and acute cardiotoxicity of ultrafine particles in young adult and old rat hearts[J]. Basic Res Cardiol, 2007,102(6):467-475. |
| [12] | Shaw CA, Robertson S, Miller MR, et al. Diesel exhaust particulate: exposed macrophages cause marked endothelial cell activation[J]. Am J Respir Cell Mol Biol, 2011,44(6):840-851. |
| [13] | Kim JB, Kim C, Choi E, et al. Particulate air pollution induces arrhythmia via oxidative stress and calcium calmodulin kinase Ⅱ activation[J]. Toxicol Appl Pharmacol, 2012,259(1):66-73. |
| [14] |
Cozzi E, Hazarika S, Stallings HW, et al. Ultrafine particulate matter exposure augments ischemia-reperfusion injury in mice[J]. Am J Physiol Heart Circ Physiol, 2006,291(2):H894-903.
pmid: 16582015 |
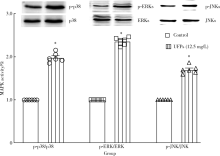
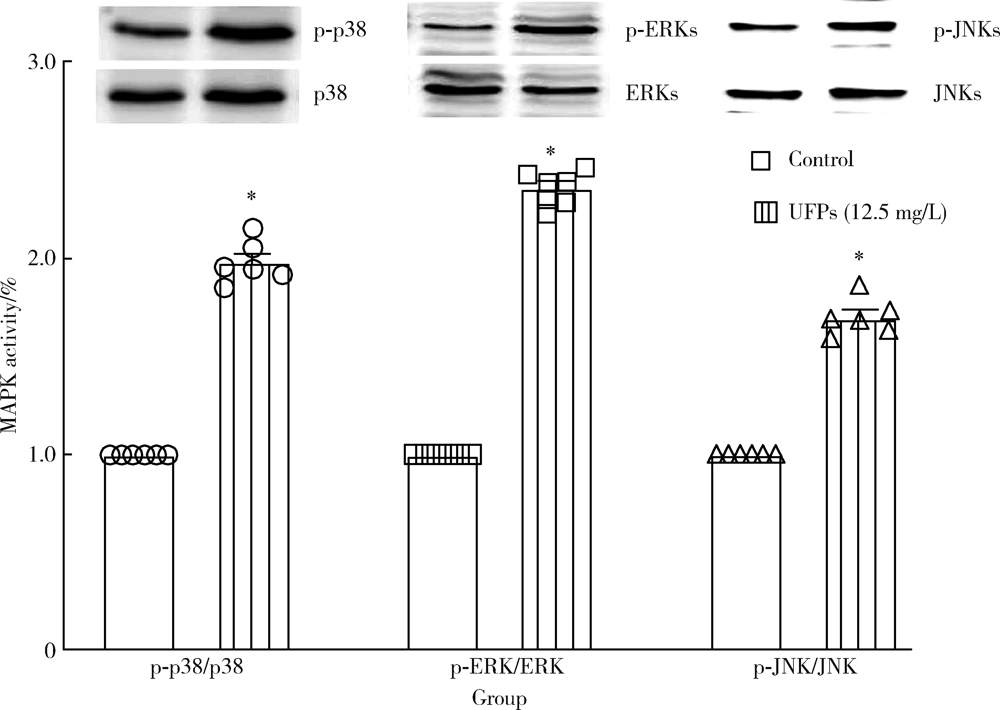
| [15] | Cao J, Qin G, Shi RZ, et al. Overproduction of reactive oxygen species and activation of MAPKs are involved in apoptosis induced by PM2.5 in rat cardiac H9C2 cells[J]. J Apple Tocicol, 2016,36(4):609-617. |
| [16] |
Baines CP, Molkentin JD. STRESS signaling pathways that modulate cardiac myocyte apoptosis[J]. J Mol Cell Cardiol, 2005,38(1):47-62.
doi: 10.1016/j.yjmcc.2004.11.004 pmid: 15623421 |
| [17] |
Zhu W, Zou Y, Aikawa R, et al. MAPK superfamily plays an important role in daunomycin-induced apoptosis of cardiac myocytes[J]. Circulation, 1999,100(20):2100-2107.
pmid: 10562267 |
| [18] |
Jarvis IW, Bergvall C, Morales DA, et al. Nanomolar levels of PAHs in extracts from urban air induce MAPK signaling in HepG2 cells[J]. Toxicol Lett, 2014,229(1):25-32.
pmid: 24910982 |
| [19] |
Rui W, Guan L, Zhang F, et al. PM2.5-induced oxidative stress increases adhesion molecules expression in human endothelial cells through the ERK/AKT/NF-κB-dependent pathway[J]. J Appl Toxicol, 2016,36(1):48-59.
pmid: 25876056 |
| [1] | Yan XUAN,Yu CAI,Xiao-xuan WANG,Qiao SHI,Li-xin QIU,Qing-xian LUAN. Effect of Porphyromonas gingivalis infection on atherosclerosis in apolipoprotein-E knockout mice [J]. Journal of Peking University (Health Sciences), 2020, 52(4): 743-749. |
| [2] | WANG Hao, CHEN Liang, YE Xiao-yun. Triptolide induces oxidative stress and apoptosis and activates PIK3/Akt signaling pathway in TM4 sertoli cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 607-612. |
| [3] | ZHU Yun-yan, LI Qian, ZHANG Yi-mei, ZHOU Yan-heng. Decreased phosphorylation of mitogen activated protein kinase and protein kinase B contribute to the inhibition of osteogenic differentiation mediated by activation of Toll like receptor in human periodontal ligament stem cells#br# [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 33-41. |
| [4] | ZHANG Yi1, SONG Xiao-ming1, ZHAO Qian, WANG Tong, Li Li-juan, CHEN Jie, XU Hong-bing, LIU Bei-bei, SUN Xiao-yan, HE Bei, HUANG Wei. Effects of exposure to ambient particulate matter and polycyclic aromatic hydrocarbons on oxidative stress biomarkers in the patients with chronic obstructive pulmonary disease [J]. Journal of Peking University(Health Sciences), 2017, 49(3): 394-402. |
| [5] | YANG Guang, CHENG Qing-li, LI Chun-lin, JIA Ya-li, YUE Wen, PEI Xue-tao, LIU Yang, ZHAO Jia-hui, DU Jing, AO Qiang-guo. High glucose reduced the repair function of kidney stem cells conditional medium to the hypoxia-injured renal tubular epithelium cells [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 125-130. |
| [6] | TU Jing-yi, ZHU Ying, SHANG Shu-ling, ZHANG Xi, TANG Hui, WANG Rui-min. Keap1-tat peptide attenuates oxidative stress damage in hippocampal CA1 region and learning and memory deficits following global cerebral ischemia [J]. Journal of Peking University(Health Sciences), 2016, 48(1): 154-159. |
| [7] | LI Peng, WAN Meng, LIU Jian-ru, LI Liang-zhong, ZHANG Da-kun. Effect of peroxisome proliferator-activated receptor-γ on endothelial cells oxidative stress induced by Porphyromonas gingivalis [J]. Journal of Peking University(Health Sciences), 2015, 47(6): 977-982. |
| [8] | LI Gang, ZHANG Hong-xian, WANG Yun-peng, ZHANG Jing,HONG Kai, TIAN Xiao-jun, MA Lu-lin. Protective effect of phloroglucinol on renal ischemia and reperfusion injury [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 743-748. |
|
||