Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (2): 270-275. doi: 10.19723/j.issn.1671-167X.2023.02.010
Previous Articles Next Articles
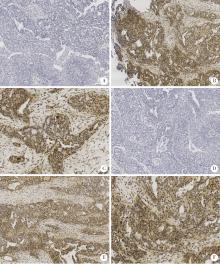
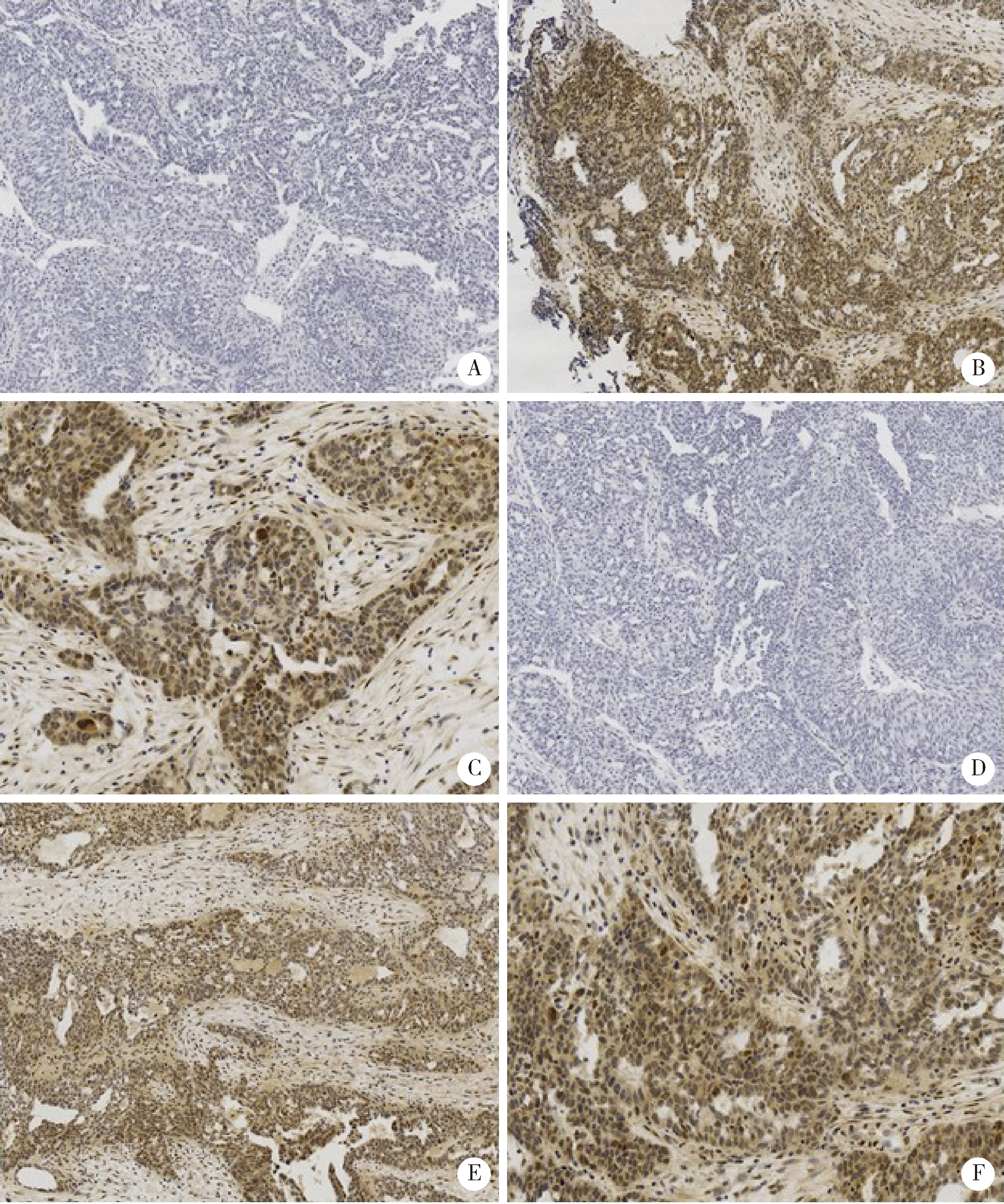
Activation of JAK/STAT in ovarian high-grade serous cancers and its prognostic significance
Jing YANG1,Juan DU1,2,Yu-xiang WANG1,2,Cong-rong LIU1,2,*( )
)
- 1. Department of Pathology, Peking University Third Hospital, Beijing 100191, China
2. Department of Pathology, Peking University School of Basic Medical Sciences, Beijing 100191, China
CLC Number:
- R737.31
| 1 |
Roeser JC , Leach SD , McAllister F . Emerging strategies for cancer immunoprevention[J]. Oncogene, 2015, 34 (50): 6029- 6039.
doi: 10.1038/onc.2015.98 |
| 2 |
Frank DA . STAT3 as a central mediator of neoplastic cellular transformation[J]. Cancer Lett, 2007, 251 (2): 199- 210.
doi: 10.1016/j.canlet.2006.10.017 |
| 3 |
Suh YA , Jo SY , Lee HY . Inhibition of IL-6/STAT3 axis and targeting Axl and Tyro3 receptor tyrosine kinases by apigenin circumvent taxol resistance in ovarian cancer cells[J]. Int J Oncol, 2015, 46 (3): 1405- 1411.
doi: 10.3892/ijo.2014.2808 |
| 4 |
Chen H , Ye D , Xie X , et al. VEGF, VEGFRs expressions and activated STATs in ovarian epithelial carcinoma[J]. Gynecol Oncol, 2004, 94 (3): 630- 635.
doi: 10.1016/j.ygyno.2004.05.056 |
| 5 |
Saini U , Naidu S , ElNaggar AC , et al. Elevated STAT3 expression in ovarian cancer ascites promotes invasion and metastasis: A potential therapeutic target[J]. Oncogene, 2017, 36 (2): 168- 181.
doi: 10.1038/onc.2016.197 |
| 6 | Perez RP , Godwin AK , Hamilton TC , et al. Ovarian cancer biology[J]. Semin Oncol, 1991, 18 (3): 186- 204. |
| 7 |
Zhang H , Liu T , Zhang Z , et al. Integrated proteogenomic characterization of human high-grade serous ovarian cancer[J]. Cell, 2016, 166 (3): 755- 765.
doi: 10.1016/j.cell.2016.05.069 |
| 8 |
Szubert S , Moszynski R , Szpurek D , et al. The expression of platelet-derived growth factor receptors (PDGFRs) and their correlation with overall survival of patients with ovarian cancer[J]. Ginekol Pol, 2019, 90 (5): 242- 249.
doi: 10.5603/GP.a2019.0045 |
| 9 |
Gadducci A , Lanfredini N , Sergiampietri C . Antiangiogenic agents in gynecological cancer: State of art and perspectives of clinical research[J]. Crit Rev Oncol Hematol, 2015, 96 (1): 113- 128.
doi: 10.1016/j.critrevonc.2015.05.009 |
| 10 |
Aravantinos G , Pectasides D . Bevacizumab in combination with chemotherapy for the treatment of advanced ovarian cancer: A systematic review[J]. J Ovarian Res, 2014, 7, 57.
doi: 10.1186/1757-2215-7-57 |
| [1] | GE Xiao-dong, LI Mei-ling, WEN Xi-lin, LI Yi, DENG Xiao-lin, WU Xiao-feng, WEN Ming, LI Shao-lin. Optimal concentration of superparamagnetic iron oxide-short hairpin RNA dual functional molecular probe transfected into ovarian cancer cells in vitro [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 754-760. |
| [2] | MA Rui-qiong, CHENG Hong-yan, YE Xue, CHEN Jun, CUI Heng, WEI Li-hui, CHANG Xiao-hong. Expression and significance of tumor necrosis factor receptor associated protein 1 in epithelial ovarian cancer [J]. Journal of Peking University(Health Sciences), 2014, 46(1): 120-124. |
|
||