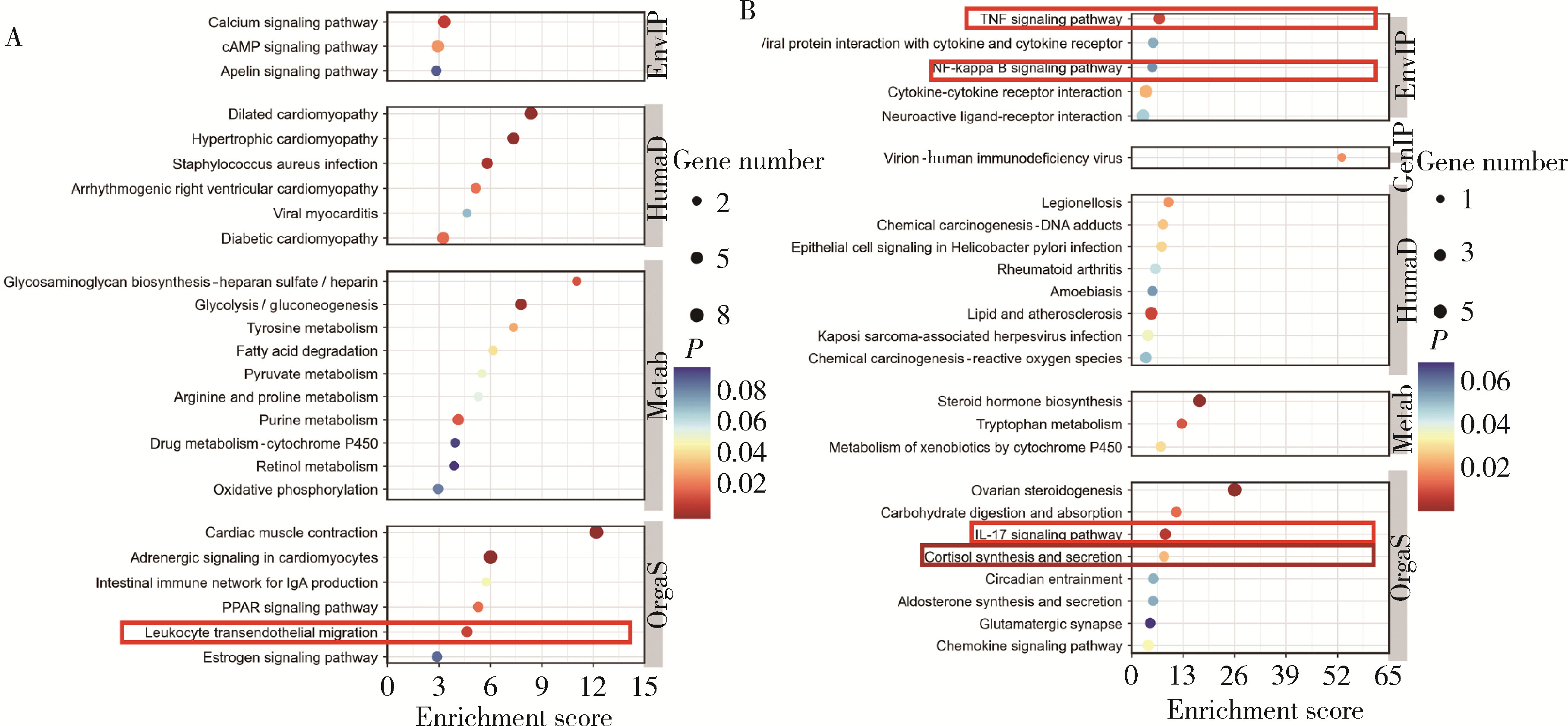
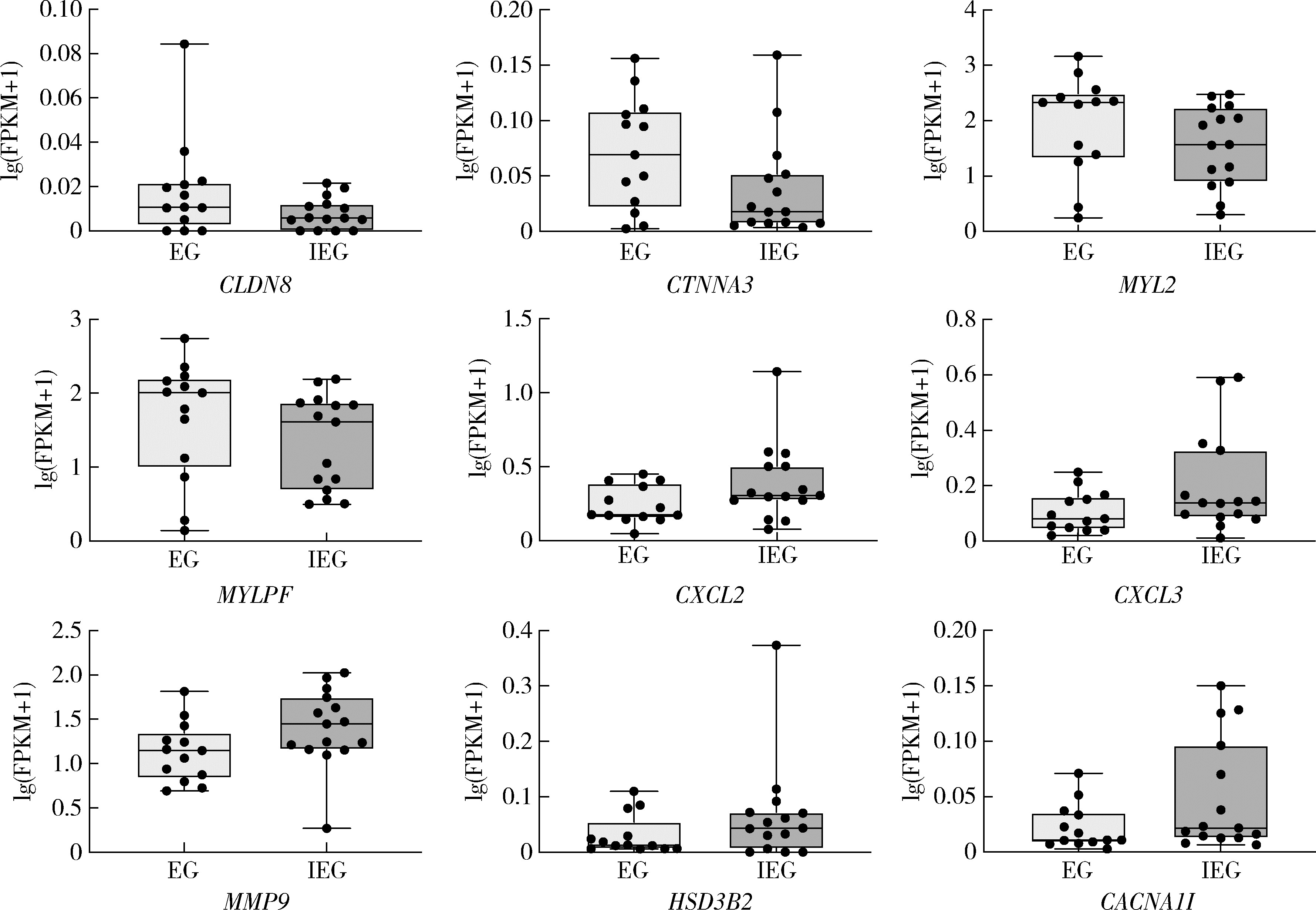
Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (1): 32-38. doi: 10.19723/j.issn.1671-167X.2024.01.006
Previous Articles Next Articles
Detection of molecular affecting sensitivity to local glucocorticoid therapy in oral lichen planus through transcriptome sequencing
Xiaomeng REN,Kaiyi LI,Chunlei LI*( )
)
- Department of Oral Medicine, Peking University School and Hospital of Stomatology & National Center of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Research Center of Oral Biomaterials and Digital Medical Devices & Beijing Key Laboratory of Digital Stomatology & NHC Research Center of Engineering and Technology for Computerized Dentistry & NMPA Key Laboratory for Dental Materials, Beijing 100081, China
CLC Number:
- R781.5
| 1 |
Li C , Tang X , Zheng X , et al. Global prevalence and incidence estimates of oral lichen planus: A systematic review and meta-analysis[J]. JAMA Dermatol, 2020, 156 (2): 172- 181.
doi: 10.1001/jamadermatol.2019.3797 |
| 2 |
Li JW , Li KY , Chan BWA , et al. Rate of malignant transformation differs based on diagnostic criteria for oral lichenoid conditions: A systematic review and meta-analysis of 24 277 patients[J]. Cancers (Basel), 2023, 15 (9): 2537.
doi: 10.3390/cancers15092537 |
| 3 |
Zhu ZD , Ren XM , Zhou MM , et al. Salivary cytokine profile in patients with oral lichen planus[J]. J Dent Sci, 2022, 17 (1): 100- 105.
doi: 10.1016/j.jds.2021.06.013 |
| 4 |
Ioannides D , Vakirlis E , Kemeny L , et al. European S1 guidelines on the management of lichen planus: A cooperation of the european dermatology forum with the european academy of dermatology and venereology[J]. J Eur Acad Dermatol Venereol, 2020, 34 (7): 1403- 1414.
doi: 10.1111/jdv.16464 |
| 5 |
中华口腔医学会口腔黏膜病学专业委员会, 中华口腔医学会中西医结合专业委员会. 口腔扁平苔藓诊疗指南(修订版)[J]. 中华口腔医学杂志, 2022, 57 (2): 115- 121.
doi: 10.3760/cma.j.cn112144-20211115-00505 |
| 6 | 左雯鑫, 李晓宇, 蔡淦英, 等. 他克莫司含漱液治疗糜烂型口腔扁平苔藓的随机单盲对照研究[J]. 上海口腔医学, 2013, 22 (6): 708- 710. |
| 7 |
Jajarm HH , Falaki F , Mahdavi O . A comparative pilot study of low intensity laser versus topical corticosteroids in the treatment of erosive-atrophic oral lichen planus[J]. Photomed Laser Surg, 2011, 29 (6): 421- 425.
doi: 10.1089/pho.2010.2876 |
| 8 |
Qing M , Zhou Y , Peng J , et al. The interleukin-6 family in tissues is closely related to the clinical outcomes of oral lichen planus[J]. J Oral Pathol Med, 2023, 52 (2): 161- 168.
doi: 10.1111/jop.13366 |
| 9 |
van der Meij EH , van der Waal I . Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications[J]. J Oral Pathol Med, 2003, 32 (9): 507- 512.
doi: 10.1034/j.1600-0714.2003.00125.x |
| 10 |
Wu Y , Xu H , Wang Y , et al. An improved scoring system for monitoring oral lichen planus: A preliminary clinical study[J]. Oral Dis, 2023, 29 (8): 3337- 3345.
doi: 10.1111/odi.14273 |
| 11 |
Hashem AS , Issrani R , Elsayed TEE , et al. Topical hyaluronic acid in the management of oral lichen planus: A comparative study[J]. J Investig Clin Dent, 2019, 10 (2): e12385.
doi: 10.1111/jicd.12385 |
| 12 |
Vilar-Villanueva M , Gándara-Vila P , Blanco-Aguilera E , et al. Psychological disorders and quality of life in oral lichen planus patients and a control group[J]. Oral Dis, 2019, 25 (6): 1645- 1651.
doi: 10.1111/odi.13106 |
| 13 |
中华口腔医学会口腔黏膜病专业委员会. 口腔扁平苔藓(萎缩型、糜烂型)疗效评价标准(试行)[J]. 中华口腔医学杂志, 2005, 40 (2): 92- 93.
doi: 10.3760/j.issn:1002-0098.2005.02.002 |
| 14 |
Bindakhil M , Akintoye S , Corby P , et al. Influence of topical corticosteroids on malignant transformation of oral lichen planus[J]. J Oral Pathol Med, 2022, 51 (2): 188- 193.
doi: 10.1111/jop.13257 |
| 15 |
Chung HS , Kim Y , Oh SJ , et al. A synthetic compound, 4-acetyl-3-methyl-6-(3, 4, 5-trimethoxyphenyl)pyrano[3, 4-c]pyran-1, 8-dione, ameliorates ovalbumin-induced asthma[J]. Bioorg Med Chem, 2013, 21 (21): 6359- 6365.
doi: 10.1016/j.bmc.2013.08.045 |
| 16 |
Chen YT , Li J , Chang JN , et al. Transcriptomic analysis of world trade center particulate matter-induced pulmonary inflammation and drug treatments[J]. Environ Int, 2023, 177, 108027.
doi: 10.1016/j.envint.2023.108027 |
| 17 | Matos Leitão M , Euclides Silva-Filho S , Arena AC , et al. Antinociceptive and anti-inflammatory properties of aqueous extract obtained from serjania marginata casar leaves[J]. J Ethnopharmacol, 2023, 304, 116018. |
| 18 |
Valdez R , Cavinder CA , Varner DD , et al. Dexamethasone downregulates expression of several genes encoding orphan nuclear receptors that are important to steroidogenesis in stallion testes[J]. J Biochem Mol Toxicol, 2019, 33 (6): e22309.
doi: 10.1002/jbt.22309 |
| 19 |
Paulusová V , Laco J , Drízhal I , et al. Expression of matrix metalloproteinase 9 in patients with oral lichen planus[J]. Acta Medica (Hradec Kralove), 2012, 55 (1): 23- 26.
doi: 10.14712/18059694.2015.70 |
| 20 | Wang H , Guan X , Luo Z , et al. The association and potentially destructive role of Th9/IL-9 is synergistic with Th17 cells by elevating mmp9 production in local lesions of oral lichen planus[J]. J Oral Pathol Med, 2018, 47 (4): 425- 433. |
| 21 | Kylmäniemi M , Oikarinen A , Oikarinen K , et al. Effects of dexamethasone and cell proliferation on the expression of matrix metalloproteinases in human mucosal normal and malignant cells[J]. J Dent Res, 1996, 75 (3): 919- 926. |
| 22 | Villa A , Sankar V , Bassani G , et al. Dexamethasone solution and dexamethasone in mucolox for the treatment of oral lichen planus: A preliminary study[J]. Oral Surg Oral Med Oral Pathol Oral Radiol, 2020, 129 (6): 585- 590. |
| [1] | Zheng-da ZHU,Yan GAO,Wen-xiu HE,Xin FANG,Yang LIU,Pan WEI,Zhi-min YAN,Hong HUA. Efficacy and safety of Nocardia rubra cell wall skeleton for the treatment of erosive oral lichen planus [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 964-969. |
| [2] | Yang LIU,Yan GAO,Xue-jie CHEN,Hong HUA. DNA cytometry of exfoliated cells in the diagnosis of oral potential malignant disorders [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 16-20. |
|
||