Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (3): 512-518. doi: 10.19723/j.issn.1671-167X.2024.03.019
Previous Articles Next Articles
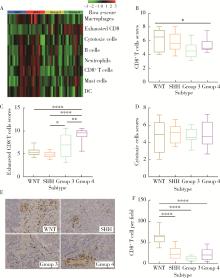
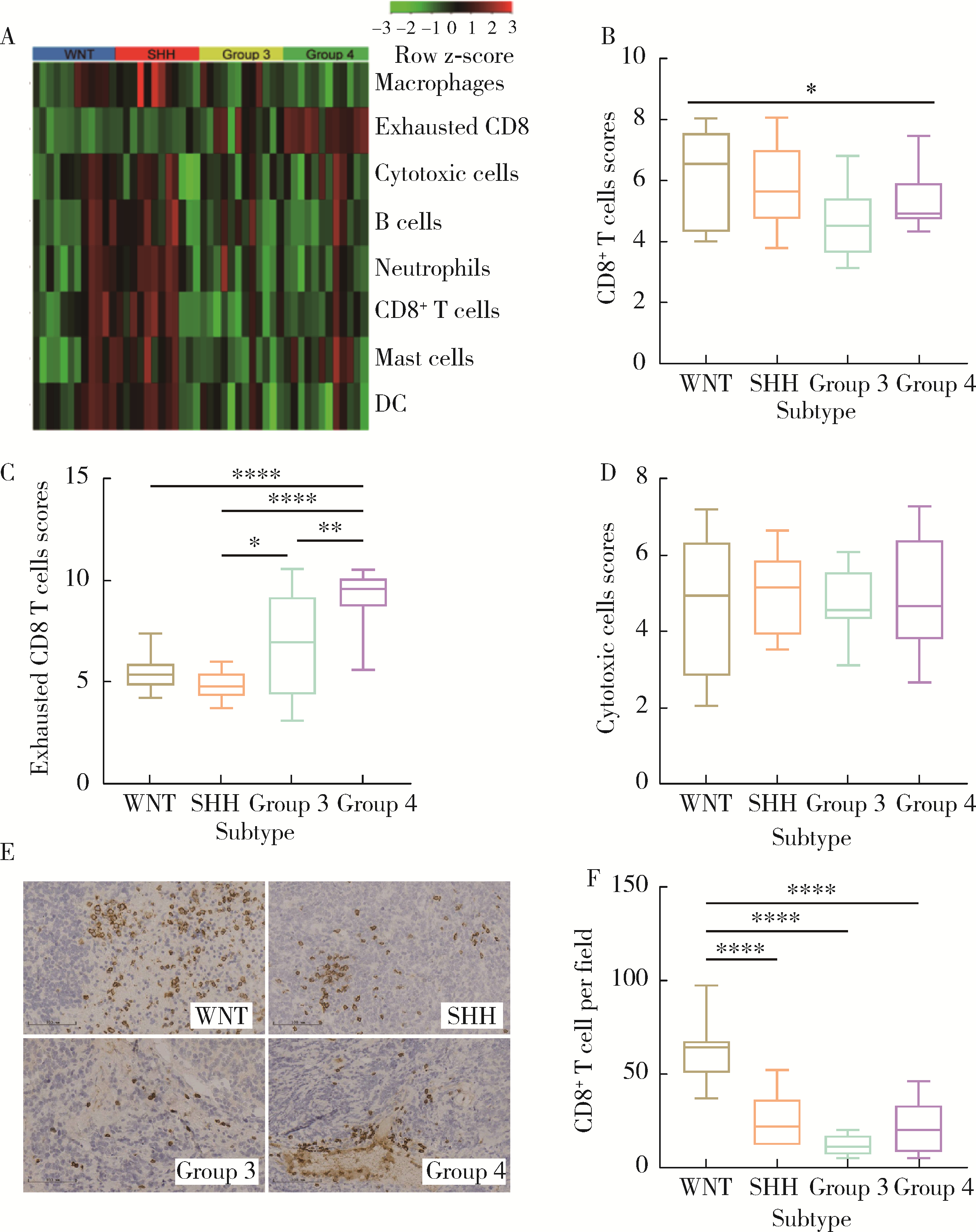
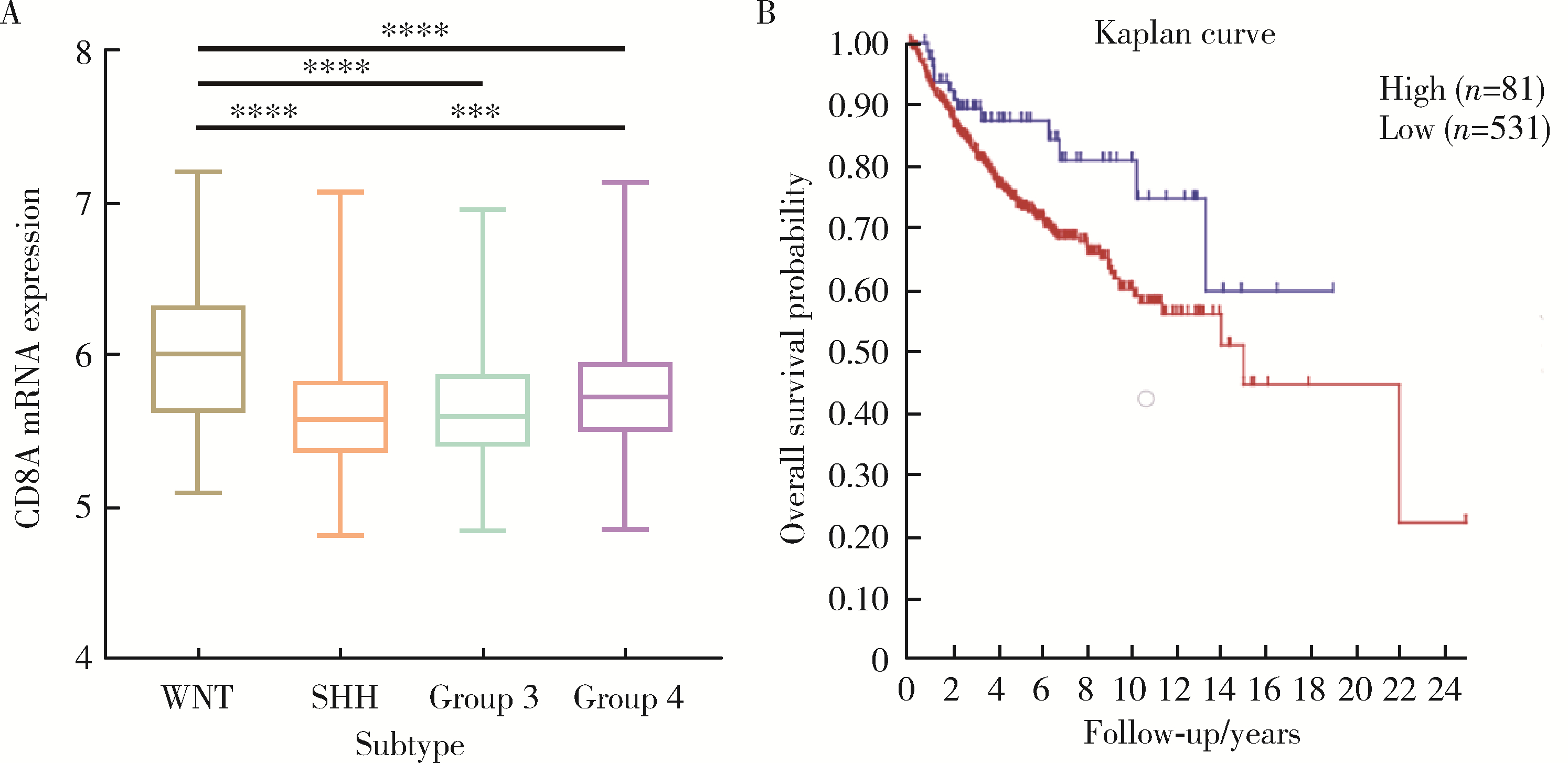
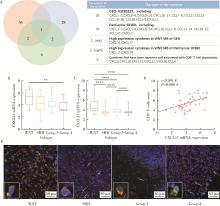
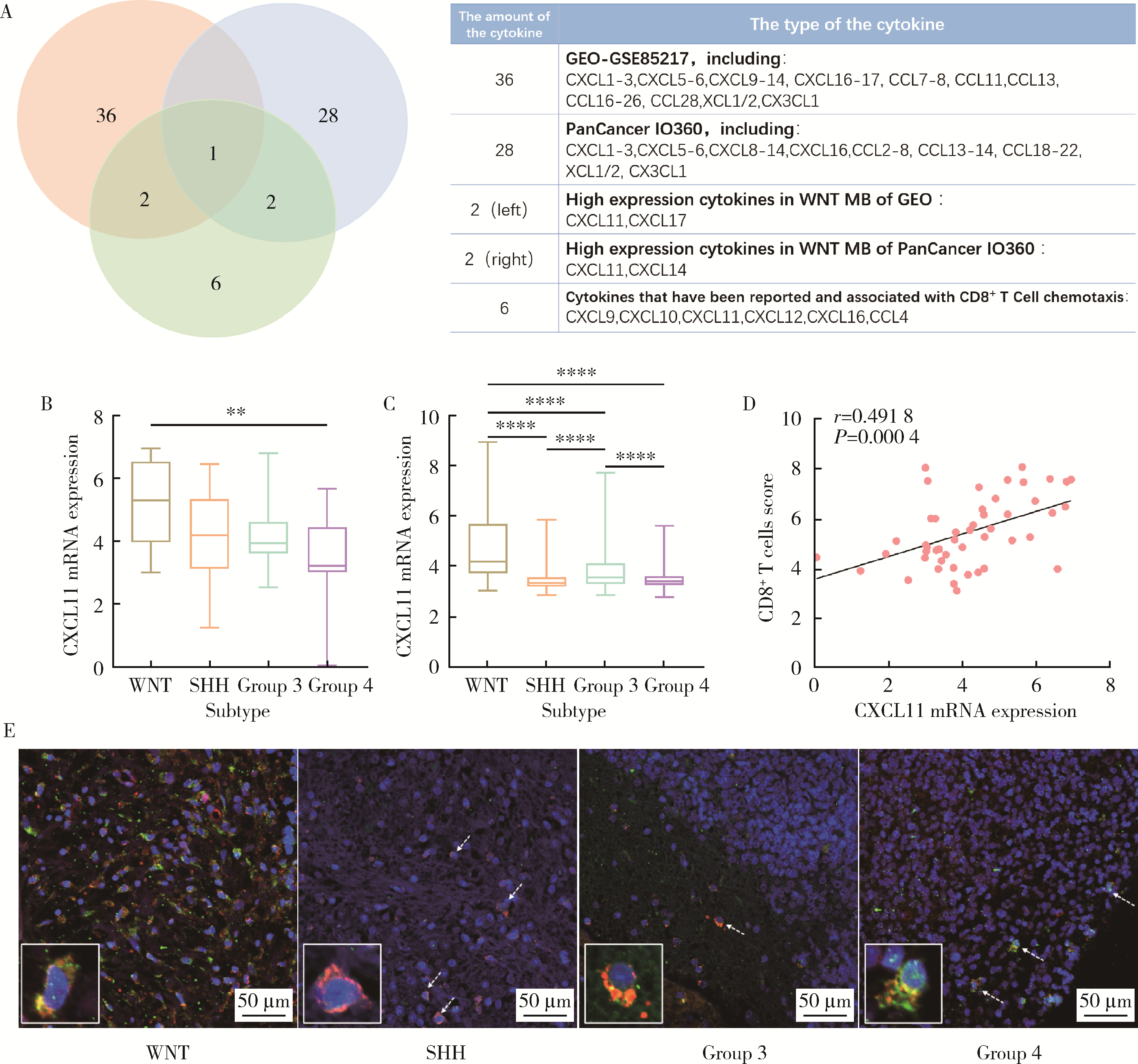
Clinicopathological characteristics of the CD8+ T lymphocytes infiltration and its mechanism in distinct molecular subtype of medulloblastoma
Xiaodong CHAI1,2,Ziwen SUN1,Haishuang LI1,Liangyi ZHU1,Xiaodan LIU1,2,Yantao LIU1,Fei PEI1,2,*( ),Qing CHANG3,*(
),Qing CHANG3,*( )
)
- 1. Department of Pathology, Peking University Third Hospital, Beijing 100191, China
2. Department of Pathology, Peking University School of Basic Medical Sciences, Beijing 100191, China
3. Department of Molecular Neuropathology, Beijing Neurosurgical Institute, Beijing 100070, China
CLC Number:
- R739.41
| 1 | Ostrom QT , Cioffi G , Gittleman H , et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2012-2016[J]. Neuro Oncol, 2019, 21 (Suppl 5): v1- v100. |
| 2 | Ostrom QT , Cioffi G , Waite K , et al. CBTRUS statistical report: Primary brain and other central nervous system tumors diagnosed in the United States in 2014-2018[J]. Neuro Oncol, 2021, 23 (12 Suppl 2): iii1- iii105. |
| 3 |
Wen PY , Packer RJ . The 2021 WHO classification of tumors of the central nervous system: Clinical implications[J]. Neuro Oncol, 2021, 23 (8): 1215- 1217.
doi: 10.1093/neuonc/noab120 |
| 4 |
Szalontay L , Khakoo Y . Medulloblastoma: An old diagnosis with new promises[J]. Curr Oncol Rep, 2020, 22 (9): 90.
doi: 10.1007/s11912-020-00953-4 |
| 5 |
Stadskleiv K , Stensvold E , Stokka K , et al. Neuropsychological functioning in survivors of childhood medulloblastoma/CNS-PNET: The role of secondary medical complications[J]. Clin Neuropsychol, 2022, 36 (3): 600- 625.
doi: 10.1080/13854046.2020.1794045 |
| 6 |
Vatner RE , Niemierko A , Misra M , et al. Endocrine deficiency as a function of radiation dose to the hypothalamus and pituitary in pediatric and young adult patients with brain tumors[J]. J Clin Oncol, 2018, 36 (28): 2854- 2862.
doi: 10.1200/JCO.2018.78.1492 |
| 7 |
Hu M , Huang L . Strategies targeting tumor immune and stromal microenvironment and their clinical relevance[J]. Adv Drug Deliv Rev, 2022, 183, 114137.
doi: 10.1016/j.addr.2022.114137 |
| 8 |
Bockmayr M , Mohme M , Klauschen F , et al. Subgroup-specific immune and stromal microenvironment in medulloblastoma[J]. Oncoimmunology, 2018, 7 (9): e1462430.
doi: 10.1080/2162402X.2018.1462430 |
| 9 |
Grabovska Y , Mackay A , O'Hare P , et al. Pediatric pan-central nervous system tumor analysis of immune-cell infiltration identifies correlates of antitumor immunity[J]. Nat Commun, 2020, 11 (1): 4324.
doi: 10.1038/s41467-020-18070-y |
| 10 |
Riemondy KA , Venkataraman S , Willard N , et al. Neoplastic and immune single-cell transcriptomics define subgroup-specific intra-tumoral heterogeneity of childhood medulloblastoma[J]. Neuro Oncol, 2022, 24 (2): 273- 286.
doi: 10.1093/neuonc/noab135 |
| 11 | Diao S , Gu C , Zhang H , et al. Immune cell infiltration and cytokine secretion analysis reveal a non-inflammatory microenvironment of medulloblastoma[J]. Oncol Lett, 2020, 20 (6): 397. |
| 12 |
Colvin RA , Campanella GS , Sun J , et al. Intracellular domains of CXCR3 that mediate CXCL9, CXCL10, and CXCL11 function[J]. J Biol Chem, 2004, 279 (29): 30219- 30227.
doi: 10.1074/jbc.M403595200 |
| 13 |
Karin N . CXCR3 ligands in cancer and autoimmunity, chemoattraction of effector T cells, and beyond[J]. Front Immunol, 2020, 11, 976.
doi: 10.3389/fimmu.2020.00976 |
| 14 |
Vollmer T , Schlickeiser S , Amini L , et al. The intratumoral CXCR3 chemokine system is predictive of chemotherapy response in human bladder cancer[J]. Sci Transl Med, 2021, 13 (576): eabb3735.
doi: 10.1126/scitranslmed.abb3735 |
| 15 |
Liu C , Zheng S , Wang Z , et al. KRAS-G12D mutation drives immune suppression and the primary resistance of anti-PD-1/PD-L1 immunotherapy in non-small cell lung cancer[J]. Cancer Commun (Lond), 2022, 42 (9): 828- 847.
doi: 10.1002/cac2.12327 |
| 16 |
Li Y , Han S , Wu B , et al. CXCL11 correlates with immune infiltration and impacts patient immunotherapy efficacy: A pan-cancer analysis[J]. Front Immunol, 2022, 13, 951247.
doi: 10.3389/fimmu.2022.951247 |
| 17 |
Chen Q , Jin J , Huang X , et al. Emp3 mediates glioblastoma-associated macrophage infiltration to drive T cell exclusion[J]. J Exp Clin Cancer Res, 2021, 40 (1): 160.
doi: 10.1186/s13046-021-01954-2 |
| 18 |
Zhu L , Yang Y , Li H , et al. Exosomal micrornas induce tumor-associated macrophages via ppargamma during tumor progression in SHH medulloblastoma[J]. Cancer Lett, 2022, 535, 215630.
doi: 10.1016/j.canlet.2022.215630 |
| 19 |
Margol AS , Robison NJ , Gnanachandran J , et al. Tumor-associa-ted macrophages in SHH subgroup of medulloblastomas[J]. Clin Cancer Res, 2015, 21 (6): 1457- 1465.
doi: 10.1158/1078-0432.CCR-14-1144 |
| 20 |
Gao Q , Wang S , Chen X , et al. Cancer-cell-secreted CXCL11 promoted CD8(+) T cells infiltration through docetaxel-induced-release of HMGB1 in NSCLC[J]. J Immunother Cancer, 2019, 7 (1): 42.
doi: 10.1186/s40425-019-0511-6 |
| 21 |
Chen Z , Liu S , He C , et al. CXCL12-CXCR4-mediated chemotaxis supports accumulation of mucosal-associated invariant T cells into the liver of patients with PBC[J]. Front Immunol, 2021, 12, 578548.
doi: 10.3389/fimmu.2021.578548 |
| 22 |
Murdamoothoo D , Sun Z , Yilmaz A , et al. Tenascin-C immobilizes infiltrating T lymphocytes through CXCL12 promoting breast cancer progression[J]. EMBO Mol Med, 2021, 13 (6): e13270.
doi: 10.15252/emmm.202013270 |
| 23 |
Di Pilato M , Kfuri-Rubens R , Pruessmann JN , et al. CXCR6 positions cytotoxic T cells to receive critical survival signals in the tumor microenvironment[J]. Cell, 2021, 184 (17): 4512- 4530. e22.
doi: 10.1016/j.cell.2021.07.015 |
| 24 |
Abdulrahman Z , Santegoets SJ , Sturm G , et al. Tumor-specific T cells support chemokine-driven spatial organization of intratumoral immune microaggregates needed for long survival[J]. J Immunother Cancer, 2022, 10 (2): e004346.
doi: 10.1136/jitc-2021-004346 |
| 25 |
Safaei S , Mohme M , Niesen J , et al. Dimeimmune: Robust estimation of infiltrating lymphocytes in CNS tumors from DNA methy-lation profiles[J]. Oncoimmunology, 2021, 10 (1): 1932365.
doi: 10.1080/2162402X.2021.1932365 |
| 26 |
Pham CD , Flores C , Yang C , et al. Differential immune microenvironments and response to immune checkpoint blockade among molecular subtypes of murine medulloblastoma[J]. Clin Cancer Res, 2016, 22 (3): 582- 595.
doi: 10.1158/1078-0432.CCR-15-0713 |
| 27 |
Liu Z , Meng Q , Bartek J Jr , et al. Tumor-infiltrating lymphocytes (TILs) from patients with glioma[J]. Oncoimmunology, 2017, 6 (2): e1252894.
doi: 10.1080/2162402X.2016.1252894 |
| 28 |
Kim AR , Choi SJ , Park J , et al. Spatial immune heterogeneity of hypoxia-induced exhausted features in high-grade glioma[J]. Oncoimmunology, 2022, 11 (1): 2026019.
doi: 10.1080/2162402X.2022.2026019 |
| 29 |
Park J , Kwon M , Kim KH , et al. Immune checkpoint inhibitor-induced reinvigoration of tumor-infiltrating CD8(+) T cells is determined by their differentiation status in glioblastoma[J]. Clin Cancer Res, 2019, 25 (8): 2549- 2559.
doi: 10.1158/1078-0432.CCR-18-2564 |
| 30 |
Salsman VS , Chow KK , Shaffer DR , et al. Crosstalk between medulloblastoma cells and endothelium triggers a strong chemotactic signal recruiting T lymphocytes to the tumor microenvironment[J]. PLoS One, 2011, 6 (5): e20267.
doi: 10.1371/journal.pone.0020267 |
| 31 |
Yi J , Shi X , Xuan Z , et al. Histone demethylase UTX/KDM6A enhances tumor immune cell recruitment, promotes differentiation and suppresses medulloblastoma[J]. Cancer Lett, 2021, 499, 188- 200.
doi: 10.1016/j.canlet.2020.11.031 |
| 32 |
Gomes-Santos IL , Amoozgar Z , Kumar AS , et al. Exercise training improves tumor control by increasing CD8+ T-cell infiltration via CXCR3 signaling and sensitizes breast cancer to immune checkpoint blockade[J]. Cancer Immunol Res, 2021, 9 (7): 765- 778.
doi: 10.1158/2326-6066.CIR-20-0499 |
| 33 |
Zheng Y , Liu Y , Zhang F , et al. Radiation combined with KRAS-MEK inhibitors enhances anticancer immunity in KRAS-mutated tumor models[J]. Transl Res, 2023, 252, 79- 90.
doi: 10.1016/j.trsl.2022.08.005 |
| 34 |
Chheda ZS , Sharma RK , Jala VR , et al. Chemoattractant receptors BLT1 and CXCR3 regulate antitumor immunity by facilitating CD8+ T cell migration into tumors[J]. J Immunol, 2016, 197 (5): 2016- 2026.
doi: 10.4049/jimmunol.1502376 |
| 35 | Wang G , Zhang Z , Zhong K , et al. CXCL11-armed oncolytic adenoviruses enhance CAR-T cell therapeutic efficacy and reprogram tumor microenvironment in glioblastoma[J]. Mol Ther, 2022, 31 (1): 134- 153. |
| [1] | Liang GENG,Jing LV,Jing FAN. Effect of Fei-Liu-Ping ointment combined with cyclophosphamide on lung cancer cell proliferation and acidic microenvironment [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 247-253. |
| [2] | FANG Wei-gang, TIAN Xin-xia. Identification of a new pro-invasion factor in tumor microenvironment: progress in function and mechanism of extracellular ATP [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 188-195. |
|
||