Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (3): 456-464. doi: 10.19723/j.issn.1671-167X.2025.03.008
Previous Articles Next Articles
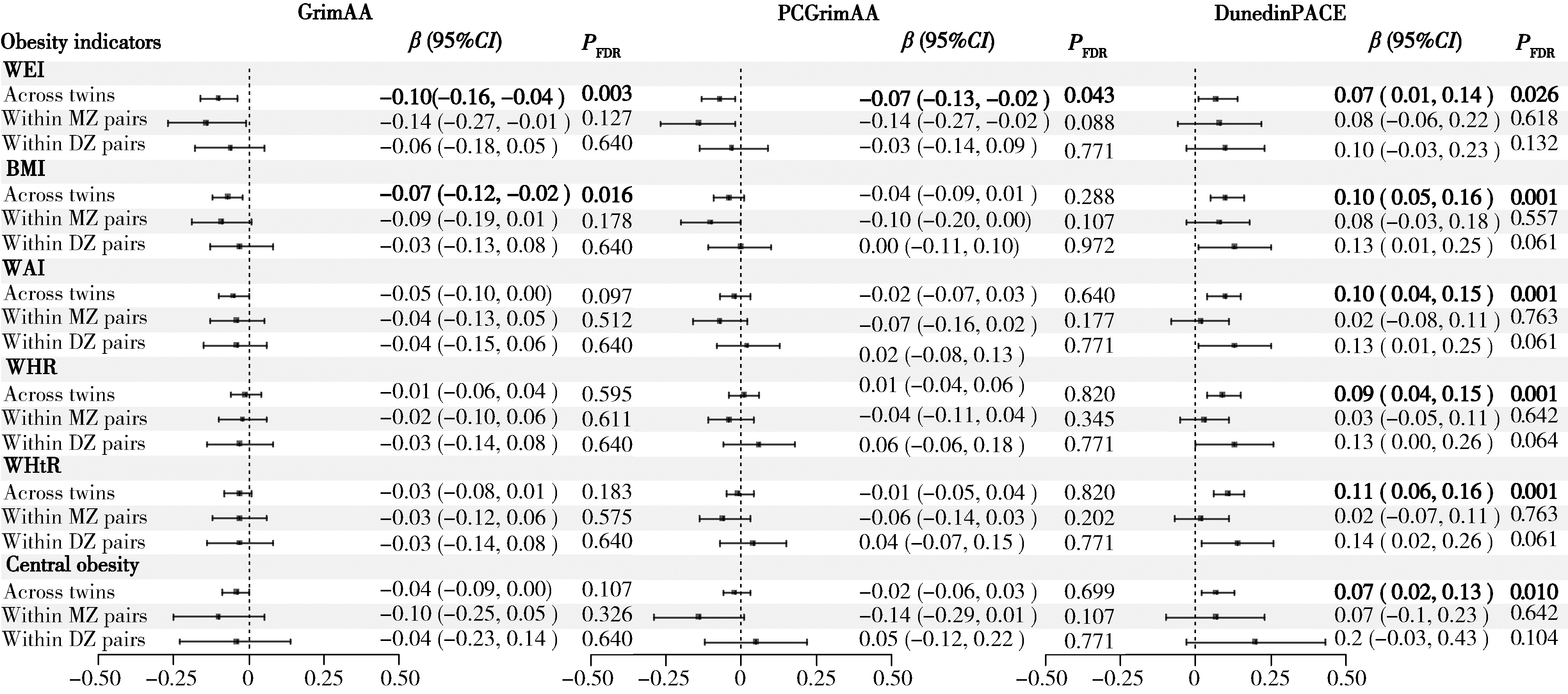
Association between DNA methylation clock and obesity-related indicators: A longitudinal twin study
Shunkai LIU1,2, Weihua CAO1,2, Jun LV1,2, Canqing YU1,2, Tao HUANG1,2, Dianjianyi SUN1,2, Chunxiao LIAO1,2, Yuanjie PANG1,2, Runhua HU1,2, Ruqin GAO3, Min YU4, Jinyi ZHOU5, Xianping WU6, Yu LIU7, Wenjing GAO1,2,*( ), Liming LI1,2
), Liming LI1,2
- 1. Department of Epidemiology and Biostatistics, Peking University School of Public Health, Beijing 100191, China
2. Key Laboratory of Epidemiology of Major Diseases (Peking University), Ministry of Education, Beijing 100191, China
3. Qingdao Center for Disease Control and Prevention, Qingdao 266033, Shandong, China
4. Zhejiang Center for Disease Control and Prevention, Hangzhou 310051, China
5. Jiangsu Center for Disease Control and Prevention, Nanjing 210008, China
6. Sichuan Center for Disease Control and Prevention, Chengdu 610041, China
7. Heilongjiang Center for Disease Control and Prevention, Harbin 150090, China
CLC Number:
- R181.33
| 1 |
doi: 10.1038/s41576-022-00511-7 |
| 2 |
doi: 10.1093/gerona/gls233 |
| 3 |
doi: 10.18632/aging.101684 |
| 4 |
doi: 10.7554/eLife.73420 |
| 5 |
doi: 10.1038/s43587-022-00248-2 |
| 6 |
doi: 10.3389/fgene.2022.819749 |
| 7 |
doi: 10.1073/pnas.2215840120 |
| 8 |
doi: 10.1111/obr.13724 |
| 9 |
doi: 10.3390/ijms23169252 |
| 10 |
doi: 10.1038/s41574-021-00551-9 |
| 11 |
doi: 10.1016/j.freeradbiomed.2018.09.039 |
| 12 |
doi: 10.1093/ajcn/nqy107 |
| 13 |
|
| 14 |
doi: 10.1002/oby.23255 |
| 15 |
doi: 10.1093/aje/kwaa251 |
| 16 |
doi: 10.1111/joim.12926 |
| 17 |
中华人民共和国卫生和计划生育委员会. WS/T428-2013成人体重判定[S]. 北京: 中国标准出版社, 2013.
|
| 18 |
doi: 10.1371/journal.pone.0123992 |
| 19 |
doi: 10.1093/gerona/glz099 |
| 20 |
doi: 10.3390/ijms20174273 |
| 21 |
doi: 10.1161/CIRCRESAHA.119.315397 |
| 22 |
doi: 10.1038/s41598-022-11562-5 |
| 23 |
doi: 10.1186/s12944-024-02042-y |
| 24 |
doi: 10.1111/joim.13528 |
| 25 |
doi: 10.1038/s41366-024-01466-x |
| 26 |
doi: 10.1016/j.arr.2011.12.003 |
| 27 |
doi: 10.1111/obr.12991 |
| 28 |
doi: 10.1113/JP271691 |
| 29 |
doi: 10.1016/j.bcp.2021.114723 |
| [1] | Zeyuan WANG, Shuanbao YU, Haoke ZHENG, Jin TAO, Yafeng FAN, Xuepei ZHANG. A preoperative prediction model for pelvic lymph node metastasis in prostate cancer: Integrating clinical characteristics and multiparametric MRI [J]. Journal of Peking University (Health Sciences), 2025, 57(4): 684-691. |
| [2] | Yihang ZHANG, Shan CAI, Ziyue CHEN, Yunfei LIU, Jiajia DANG, Di SHI, Jiaxin LI, Tianyu HUANG, Yi SONG. Establishment of outcome indicators for the implementation of comprehensive intervention for multimorbidity of myopia and obesity among children and adolescents based on the RE-AIM framework [J]. Journal of Peking University (Health Sciences), 2025, 57(3): 436-441. |
| [3] | Ping LI, Haixue WANG, Xiao GAO, Yajing HAN, Hui WANG, Haijun WANG, Yingying MU. A randomized controlled trial of weight management based on mobile health techno-logy among overweight or obese pregnant women [J]. Journal of Peking University (Health Sciences), 2025, 57(3): 465-472. |
| [4] | Jianjun SUN, Qianquan MA, Xiaoliang YIN, Chenlong YANG, Jia ZHANG, Suhua CHEN, Chao WU, Jingcheng XIE, Yunfeng HAN, Guozhong LIN, Yu SI, Jun YANG, Haibo WU, Qiang ZHAO. Significance of precise classification of sacral meningeal cysts by multiple dimensions radiographic reconstruction MRI in guiding operative strategy and rehabilitation [J]. Journal of Peking University (Health Sciences), 2025, 57(2): 303-308. |
| [5] | Yujia ZHU, Hua SHEN, Aonan WEN, Zixiang GAO, Qingzhao QIN, Shenyao SHAN, Wenbo LI, Xiangling FU, Yijiao ZHAO, Yong WANG. Deep learning algorithms for intelligent construction of a three-dimensional maxillofacial symmetry reference plane [J]. Journal of Peking University (Health Sciences), 2025, 57(1): 113-120. |
| [6] | Huili LIU, Bei WEN, Xue BAI, Ming'an CHEN, Min LI. Association between weight-adjusted waist index and pain: A cross-sectional study [J]. Journal of Peking University (Health Sciences), 2025, 57(1): 178-184. |
| [7] | Wenxin CAI, Qiongying YANG, Dan HAN, Zhe CHEN, Yongjing CHENG. Application and prospects of infrared thermography in rheumatic diseases [J]. Journal of Peking University (Health Sciences), 2024, 56(6): 1132-1136. |
| [8] | Yuxuan TIAN,Mingjian RUAN,Yi LIU,Derun LI,Jingyun WU,Qi SHEN,Yu FAN,Jie JIN. Predictive effect of the dual-parametric MRI modified maximum diameter of the lesions with PI-RADS 4 and 5 on the clinically significant prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 567-574. |
| [9] | Jing CHEN,Rui SHAN,Wucai XIAO,Xiaorui ZHANG,Zheng LIU. Association between self-control and co-occurrence of depressive symptoms and overweight or obesity during adolescence and early adulthood: A ten-year prospective cohort study based on national surveys [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 397-402. |
| [10] | Xiaotong LING,Liuyang QU,Danni ZHENG,Jing YANG,Xuebing YAN,Denggao LIU,Yan GAO. Three-dimensional radiographic features of calcifying odontogenic cyst and calcifying epithelial odontogenic tumor [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 131-137. |
| [11] | Chu-yun CHEN,Peng-fei SUN,Jing ZHAO,Jia JIA,Fang-fang FAN,Chun-yan WANG,Jian-ping LI,Yi-meng JIANG,Yong HUO,Yan ZHANG. Related factors of endogenous erythropoietin and its association with 10-year risks of cardiovascular disease in a community-based Chinese study [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1068-1073. |
| [12] | Yi LIU,Chang-wei YUAN,Jing-yun WU,Qi SHEN,Jiang-xi XIAO,Zheng ZHAO,Xiao-ying WANG,Xue-song LI,Zhi-song HE,Li-qun ZHOU. Diagnostic efficacy of prostate cancer using targeted biopsy with 6-core systematic biopsy for patients with PI-RADS 5 [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 812-817. |
| [13] | Chang-wei YUAN,De-run LI,Zhi-hua LI,Yi LIU,Gang-zhi SHAN,Xue-song LI,Li-qun ZHOU. Application of dynamic contrast enhanced status in multiparametric magnetic resonance imaging for prostatic cancer with PI-RADS 4 lesion [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 838-842. |
| [14] | Zhan-yi ZHANG,Fan ZHANG,Ye YAN,Cai-guang CAO,Chang-jian LI,Shao-hui DENG,Yue-hao SUN,Tian-liang HUANG,Yun-he GUAN,Nan LI,Min LU,Zhen-hua HU,Shu-dong ZHANG. Near-infrared targeted probe designed for intraoperative imaging of prostatic neurovascular bundles [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 843-850. |
| [15] | Zhuo-hua LIN,Ru-yi CAI,Yang SUN,Rong MU,Li-gang CUI. Methodology and clinical use of superb microvascular imaging in assessing micro-circulation changes of fingertips in systemic sclerosis [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 636-640. |
|
||