Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (4): 764-771. doi: 10.19723/j.issn.1671-167X.2025.04.022
Previous Articles Next Articles
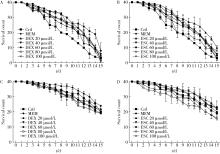
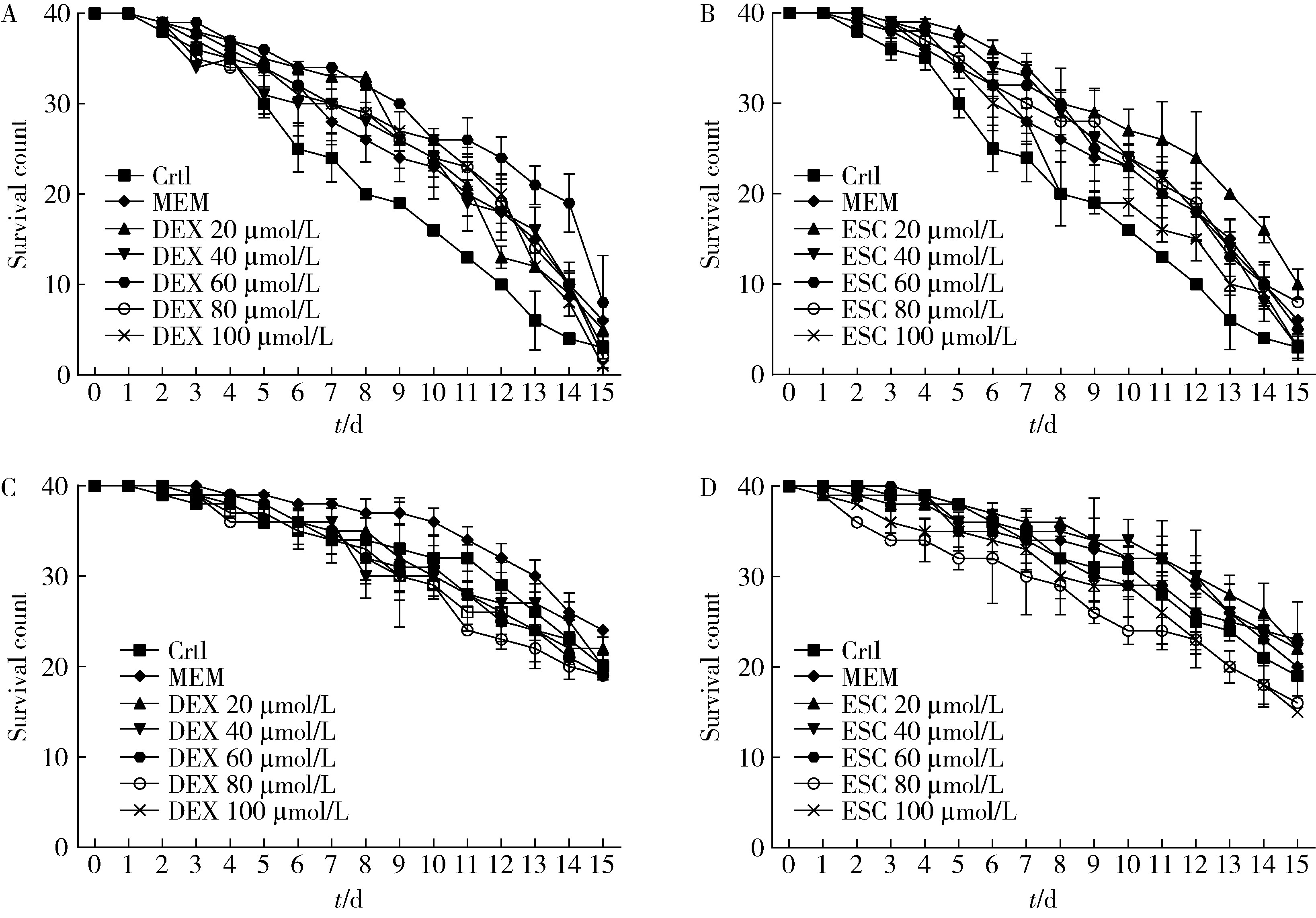
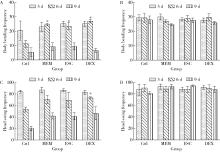
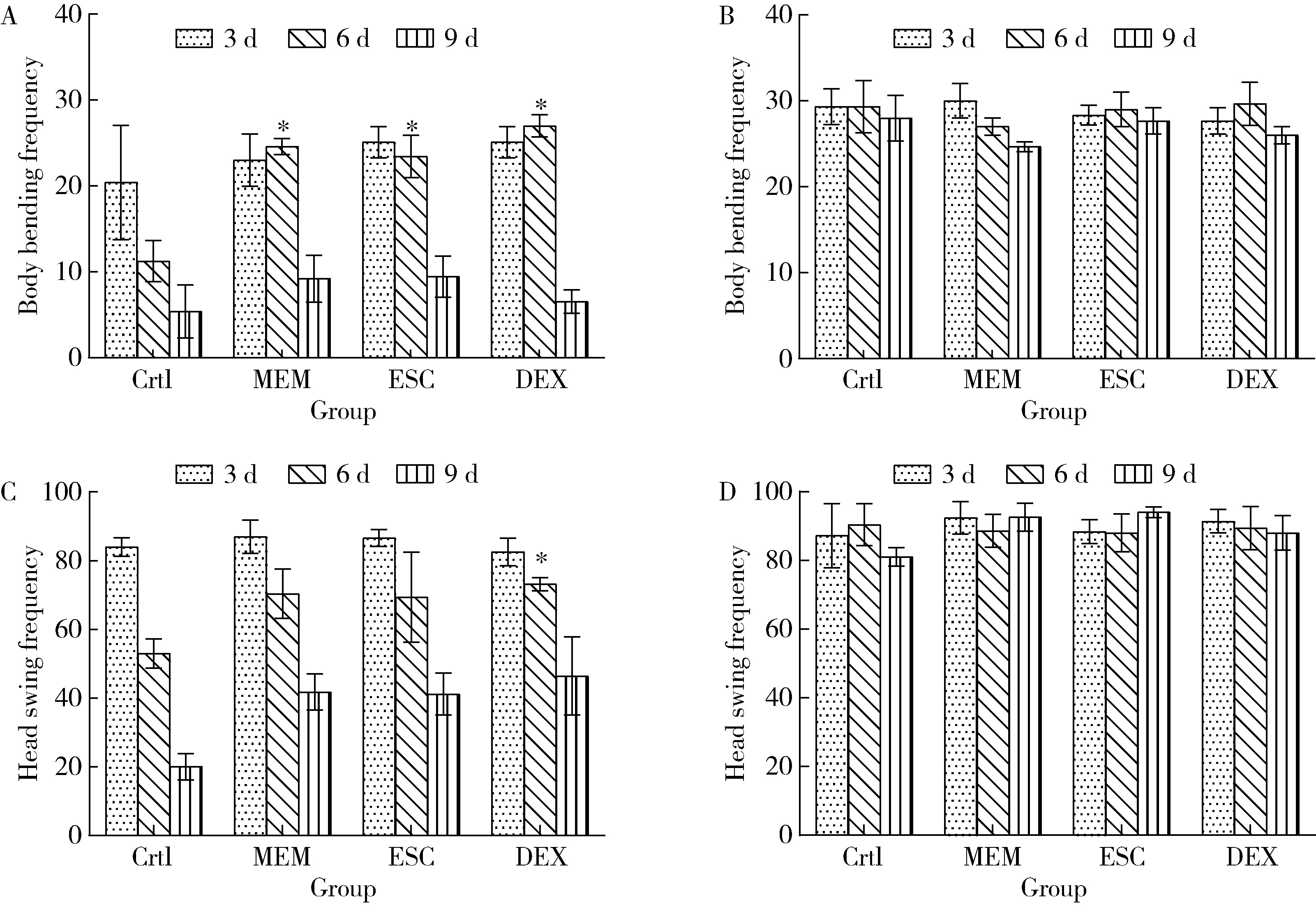
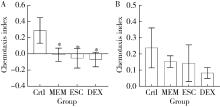
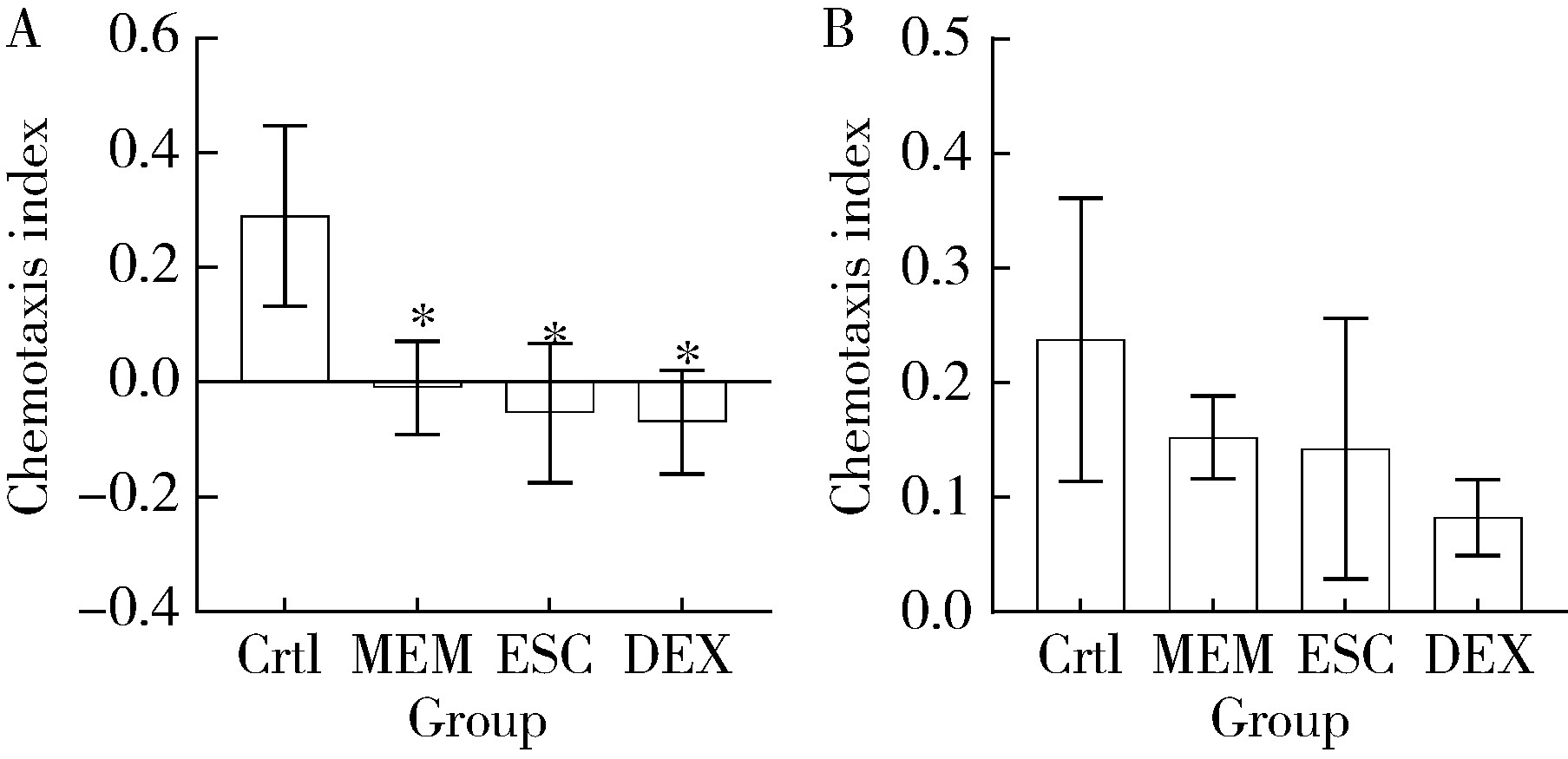
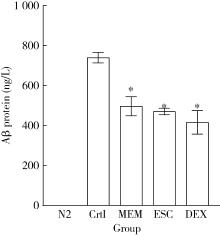
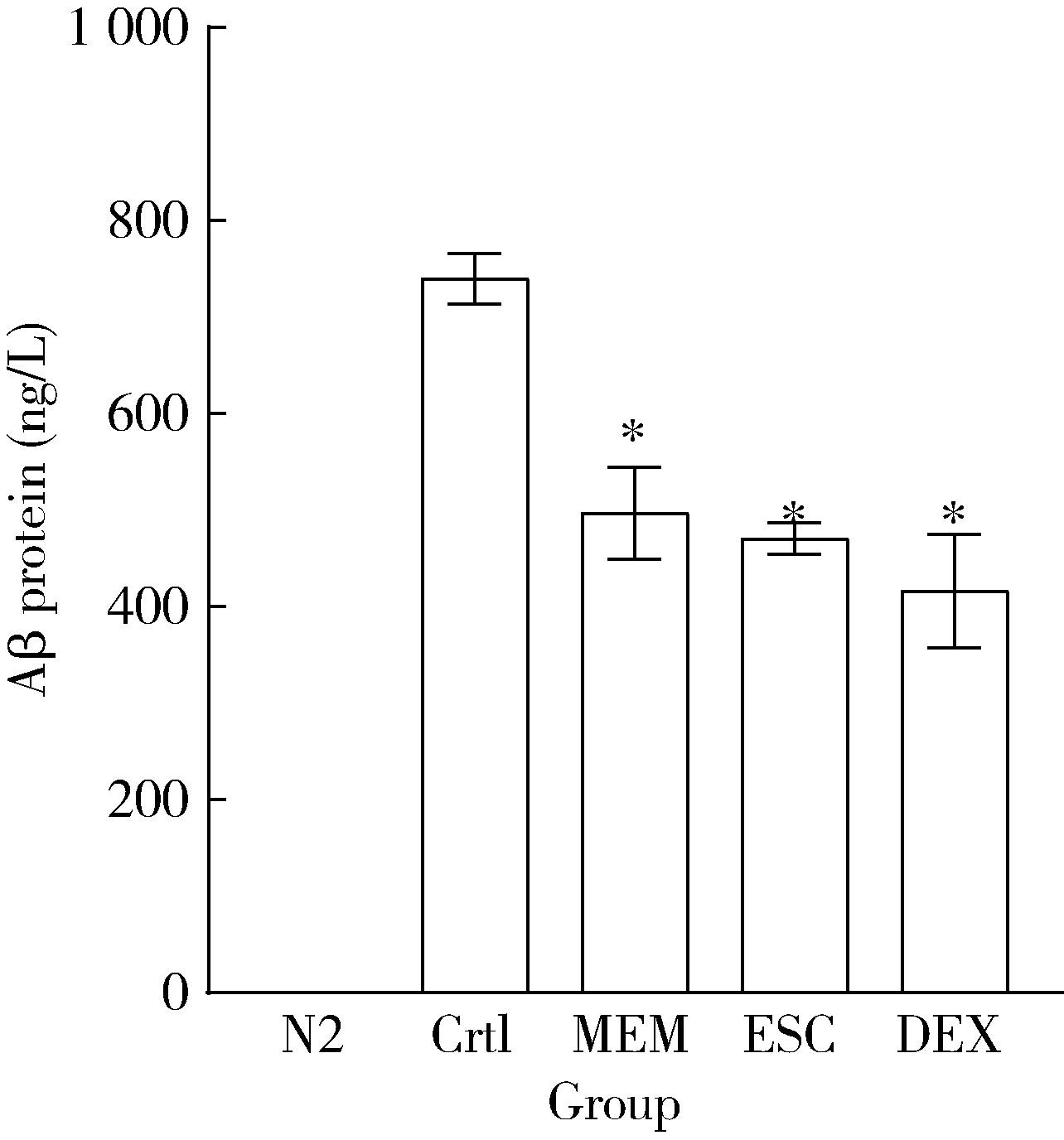
Protective effects of escin and dextromethorphan on Alzheimer disease in Caenorhabditis elegans models
Yiping ZHANG1, Ludi LI1, An ZHU2, Wusheng XIAO1, Qi WANG1,*( )
)
- 1. Department of Toxicology, Peking University School of Public Health, Key Laboratory of State Administration of Traditional Chinese Medicine for Compatibility Toxicology, Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety, Beijing 100191, China
2. Key Laboratory of Gastrointestinal Malignant Tumors, Basic Medical College, Fujian Medical University, Ministry of Education, Fuzhou 350108, China
CLC Number:
- R749.16
| 1 |
|
| 2 |
|
| 3 |
|
| 4 |
|
| 5 |
周小涛, 杨梦艳, 张继川. 细胞自噬与阿尔茨海默病的关系[J]. 中国组织化学与细胞化学杂志, 2022, 31 (5): 517- 522.
|
| 6 |
陈长军, 徐为华, 张梦晨, 等. 七叶皂苷药理活性及制剂临床应用研究进展[J]. 武汉大学学报(医学版), 2020, 41 (1): 157- 163.
|
| 7 |
|
| 8 |
徐效凤. 右美沙芬在血管性痴呆中的保护作用及机制研究[D], 上海: 上海交通大学, 2015.
|
| 9 |
|
| 10 |
|
| 11 |
|
| 12 |
|
| 13 |
|
| 14 |
|
| 15 |
|
| 16 |
|
| 17 |
|
| 18 |
|
| 19 |
李品洁. 线虫头部运动: 定量分析和神经机制研究[D], 合肥: 中国科学技术大学, 2022.
|
| 20 |
|
| 21 |
|
| 22 |
徐玉兰, 薛雅丹, 康利军. 秀丽隐杆线虫神经胶质细胞对神经系统发育和功能的影响[J]. 浙江大学学报(医学版), 2016, 45 (3): 315- 322.
|
| 23 |
|
| 24 |
|
| 25 |
邹羽真, 韩菲, 梅丹. 右美沙芬在神经系统应用的研究新进展[J]. 中国医院药学杂志, 2019, 39 (3): 313- 317.
|
| [1] | Lijie HE,Chunyan ZHANG,Jing WANG. Expression relationship and significance of NEAT1 and miR-27a-3p in serum and cerebrospinal fluid of patients with Alzheimer disease [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 207-212. |
|
||