1 资料与方法
1.1 研究对象
1.2 数据收集
1.3 指标定义
1.4 统计学分析
2 结果
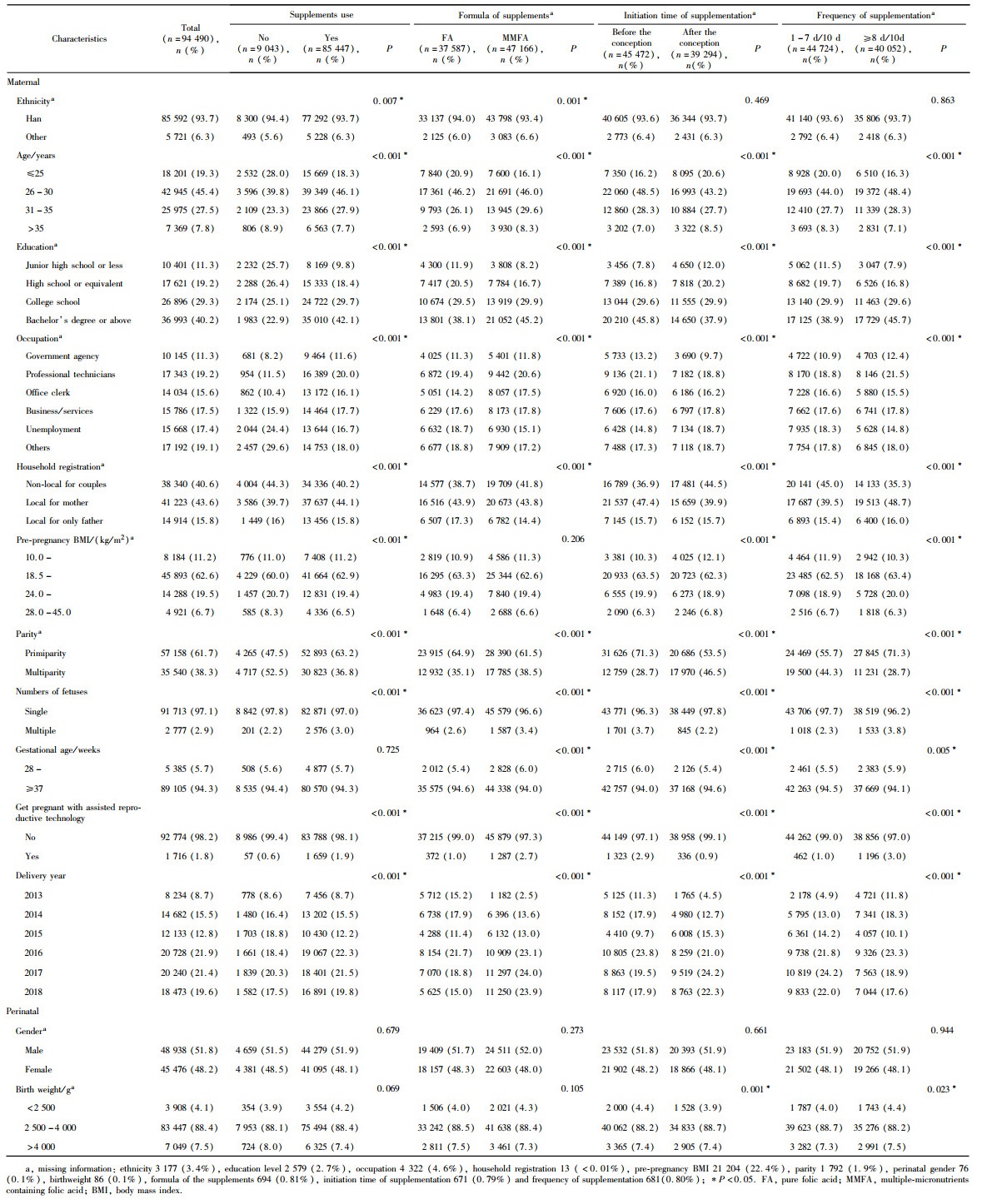
2.1 研究对象基本特征
表1 叶酸营养素补充剂不同比对组研究对象基本特征Table 1 Maternal and perinatal characteristics among the comparison groups of FA or MMFA |
 |
2.2 母亲围受孕期服用叶酸营养素补充剂与围产儿死亡率的关系
表2 母亲围受孕期服用营养补充剂与围产儿死亡率关系Table 2 Association between maternal periconceptional supplementation of FA or MMFA and risk for PMR |
| n | PM, n(‰) | cRR (95%CI) | aRR (95%CI)a | aRR (95%CI)b | |
| FA/MMFA supplementation | |||||
| No use | 9 043 | 31 (3.43) | 1 | 1 | 1 |
| Use | 85 447 | 225 (2.63) | 0.77 (0.54-1.14) | 0.89 (0.75-1.05) | 0.93 (0.77-1.13) |
| Formula of the supplements | |||||
| FA | 37 587 | 84 (2.23) | 1 | 1 | 1 |
| MMFA | 47 166 | 141 (2.99) | 1.34 (1.02-1.76) | 1.29 (1.07-1.56) | 1.31 (1.06-1.62) |
| Initiation time of supplementation | |||||
| No use | 9 043 | 31 (3.43) | 1 | 1 | 1 |
| Post-conception | 39 294 | 112 (2.85) | 0.83 (0.57-1.26) | 0.94 (0.74-1.18) | 0.95 (0.73-1.22) |
| Pre-conception | 45 472 | 113 (2.49) | 0.72 (0.49-1.10) | 0.87 (0.69-1.09) | 0.89 (0.68-1.15) |
| Frequency of supplementation | |||||
| No use | 9 043 | 31 (3.43) | 1 | 1 | 1 |
| 1-7 d/10 d | 44 724 | 126 (2.82) | 0.82 (0.56-1.24) | 0.94 (0.75-1.17) | 0.98 (0.77-1.25) |
| ≥8 d/10 d | 40 052 | 99 (2.47) | 0.72 (0.49-1.10) | 0.84 (0.66-1.07) | 0.84 (0.63-1.11) |
a, adjusted for ethnicity, age, education level, occupation, household registration, parity, numbers of fetuses, gestational age, pregnant with assisted reproductive technology and delivery year; b, adjusted for ethnicity, age, education level, occupation, household registration, parity, numbers of fetuses, gestational age, pregnant with assisted reproductive technology, delivery year and pre-pregnancy BMI. FA, pure folic acid; MMFA, multiple-micronutrients containing folic acid; PM, perinatal mortality; PMR, perinatal mortality rate; cRR, crude risk ratio; aRR, adjusted risk ratio; BMI, body mass index. |
2.3 母亲围受孕期服用叶酸营养素补充剂和孕早期血浆Hcy浓度的关系
表3 围受孕期服用叶酸营养素补充剂与孕早期血浆Hcy浓度的关系Table 3 Relationship between maternal periconceptional supplementation of FA or MMFA and plasma Hcy concentration in the first trimester of pregnant period |
| Status of supplementation | n | Hcy/(μmol/L), M (P25, P75) | P |
| FA/MMFA supplementation | <0.001 | ||
| No use | 1 427 | 6.5 (5.6, 7.7) | |
| Use | 15 627 | 6.1 (5.3, 7.0) | |
| Formula of the supplements | <0.001 | ||
| FA | 5 853 | 6.2 (5.4, 7.2) | |
| MMFA | 9 757 | 6.0 (5.3, 6.9) | |
| Initiation time of supplementation | <0.001 | ||
| Pre-conception | 7 500 | 6.0 (5.3, 6.9) | |
| Post-conception | 8 124 | 6.2 (5.4, 7.2) | |
| Frequency of supplementation | <0.001 | ||
| 1-7 d/10 d | 8 889 | 6.2 (5.4, 7.2) | |
| ≥8 d/10 d | 6 733 | 6.0 (5.3, 6.9) |
Hcy, homocysteine; FA, pure folic acid; MMFA, multiple-micronutrients containing folic acid. |
