1 资料与方法
1.1 研究对象
1.2 资料收集
1.3 统计学分析
2 结果
2.1 45例TFE3-RCC患者基线特征总体比较
表1 45例TFE3重排肾细胞癌患者(合并或不合并静脉癌栓)的基线特征Table 1 Baseline characteristics of 45 TFE3 -RCC patients with or without VT |
| Items | TFE3-VT (n=13) | TFE3-non-VT (n=32) | P value |
| Age/years, ${\bar x}$±s | 37.9 ±14.4 | 36.0 ±13.1 | 0.662 |
| Gender, n (%) | 0.638 | ||
| Male | 4 (30.8) | 14 (43.8) | |
| Female | 9 (69.2) | 18 (56.2) | |
| BMI/(kg/m2), ${\bar x}$±s | 22.2 ±3.5 | 24.8 ±3.4 | 0.023* |
| aCCI, M (P25, P75) | 1.0 (0.0, 1.0) | 0.0 (0.0, 1.0) | 0.376 |
| ASA level, n (%) | 0.840 | ||
| 1 | 7 (53.8) | 20 (62.5) | |
| 2 | 6 (46.2) | 12 (37.5) | |
| Cr/(μmoL/L), ${\bar x}$±s | 75.3 ±15.9 | 75.6±16.6 | 0.953 |
| Laterality, n (%) | 0.924 | ||
| Left | 7 (53.8) | 15 (46.9) | |
| Right | 6 (46.2) | 17 (53.1) | |
| Surgical approach, n (%) | < 0.001* | ||
| Laparoscopic | 2 (15.4) | 23 (71.9) | |
| Open | 6 (46.1) | 0 (0.0) | |
| Robotic | 5 (38.5) | 9 (28.1) | |
| Operative time/min, M (P25, P75) | 292.0 (194.0, 320.0) | 138.0 (109.8, 167.8) | < 0.001* |
| Blood loss/mL, M (P25, P75) | 600.0 (300.0, 1 100.0) | 40.0 (17.5, 100.0) | < 0.001* |
| RBC transfusion/mL, M (P25, P75) | 0.0 (0.0, 600.0) | 0.0 (0.0, 0.0) | < 0.001* |
| Clavien-Dindo level, n (%) | 0.031* | ||
| 0-Ⅰ | 9 (69.2) | 31 (96.9) | |
| Ⅱ | 4 (30.8) | 1 (3.1) | |
| Postoperative hospital stays/d, M (P25, P75) | 9.0 (6.0, 14.0) | 6.0 (5.0, 6.0) | 0.009* |
| Tumor diameter/cm, M (P25, P75) | 10.3 (9.6, 12.0) | 4.4 (3.2, 6.5) | < 0.001* |
| pT stage, n (%) | < 0.001* | ||
| pT1 | 0 (0.0) | 23 (71.9) | |
| pT2 | 0 (0.0) | 4 (12.5) | |
| pT3 | 11 (84.6) | 5 (15.6) | |
| pT4 | 2 (15.4) | 0 (0.0) | |
| N1 stage, n (%) | 5 (38.5) | 3 (9.4) | 0.060 |
| M1 stage, n (%) | 6 (46.2) | 0 (0.0) | < 0.001* |
| Clinical tumor stage, n (%) | < 0.001* | ||
| Stage Ⅰ | 0 (0.0) | 22 (68.8) | |
| Stage Ⅱ | 0 (0.0) | 4 (12.5) | |
| Stage Ⅲ | 6 (46.2) | 6 (18.8) | |
| Stage Ⅳ | 7 (53.8) | 0 (0.0) | |
| Nuclear grade, n (%) | 0.003* | ||
| Ⅰ-Ⅱ | 0 (0.0) | 17 (53.1) | |
| Ⅲ-Ⅳ | 13 (100.0) | 15 (46.9) | |
| Sarcomatoid differentiation, n (%) | 2 (15.4) | 1 (3.1) | 0.404 |
*P < 0.05. TFE3 -RCC, TFE3 -rearranged renal cell carcinoma; VT, venous tumor thrombus; TFE3 -VT, TFE3 -RCC patients with VT; TFE3 -non-VT, TFE3 -RCC patients without VT; BMI, body mass index; aCCI, age-adjusted Charlson comorbidity index; ASA, American Society of Anesthesiologists; Cr, serum creatinine; RBC, red blood cells. |
2.2 13例TFE3 -VT患者的临床及病理特征
2.2.1 临床病史、影像学表现及手术情况
表2 13例TFE3-VT患者的临床病史、肿瘤影像学特征及手术资料Table 2 Clinical history, radiological tumor characteristics and surgical data of 13 TFE3-VT patients |
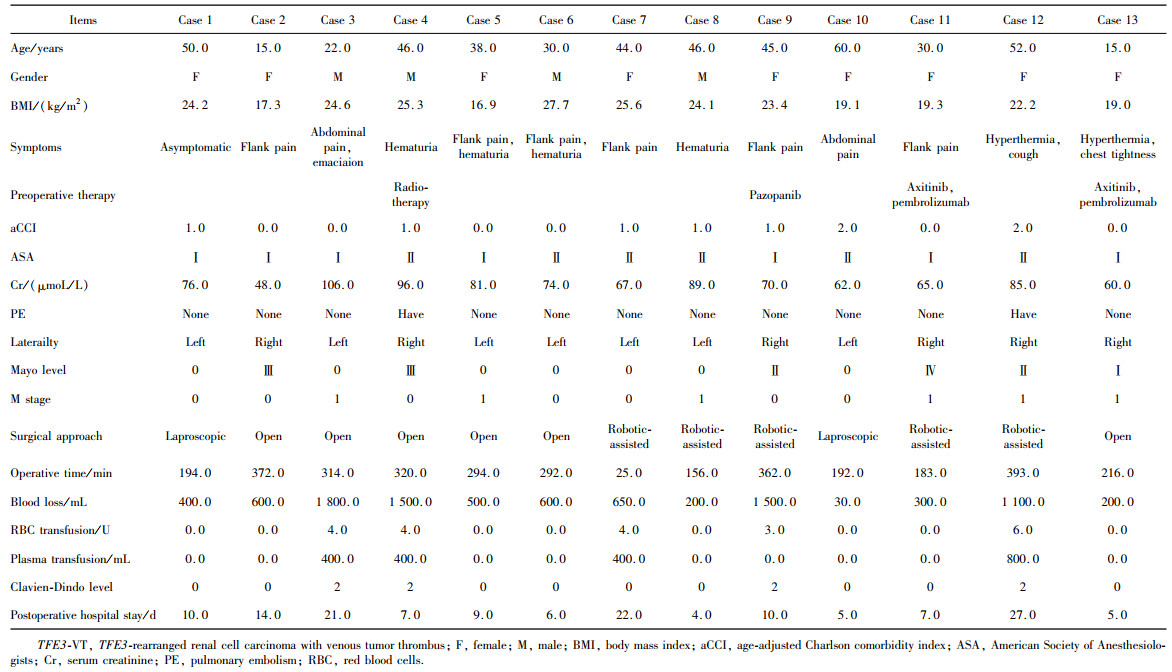 |
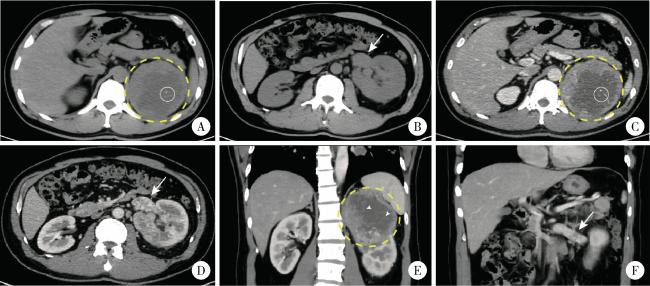
图1 1例合并Mayo 0级肾静脉癌栓的TFE3重排肾细胞癌患者(病例8)的增强CT影像Figure 1 Contrast-enhanced CT images from a left-sided TFE3 -rearranged renal cell carcinoma patient combined with Mayo level 0 renal vein thrombus (case 8) A, B, 9.4×9.6×9.5 cm mass was identified in the upper pole of the left kidney, the margin of the mass was well-defined; on non-contrast CT scan, the mass (yellow circle with dashed line) demonstrated heterogeneous soft tissue density (50-70 HU) with patchy areas of low density (30-40 HU); central punctate calcifications were observed within the lesion (white circle with solid line); the left renal vein was dilated (white arrow, axial plane). C, D, on contrast-enhanced CT imaging (arterial phase), the solid components of the mass showed marked heterogeneous enhancement (80-120 HU), while the cystic areas within the lesion exhibited no obvious enhancement; tumor thrombus was identified within the renal vein (white arrow), measuring approximately 3.7 cm in length (axial plane). E, F, on contrast-enhanced CT imaging (arterial phase), multiple fine septations were visible within the cystic areas and demonstrated enhancement (white arrow head); the perirenal fascia was compressed, and the lesion was in close proximity to the left adrenal gland and spleen; the left renal vein exhibited a filling defect and indicated tumor thrombus (white arrow); no significantly enlarged lymph nodes were seen (coronal plane). |
2.2.2 组织病理学特征
表3 13例TFE3-VT患者的组织病理学特征Table 3 Histopathological features of 13 TFE3-VT patients |
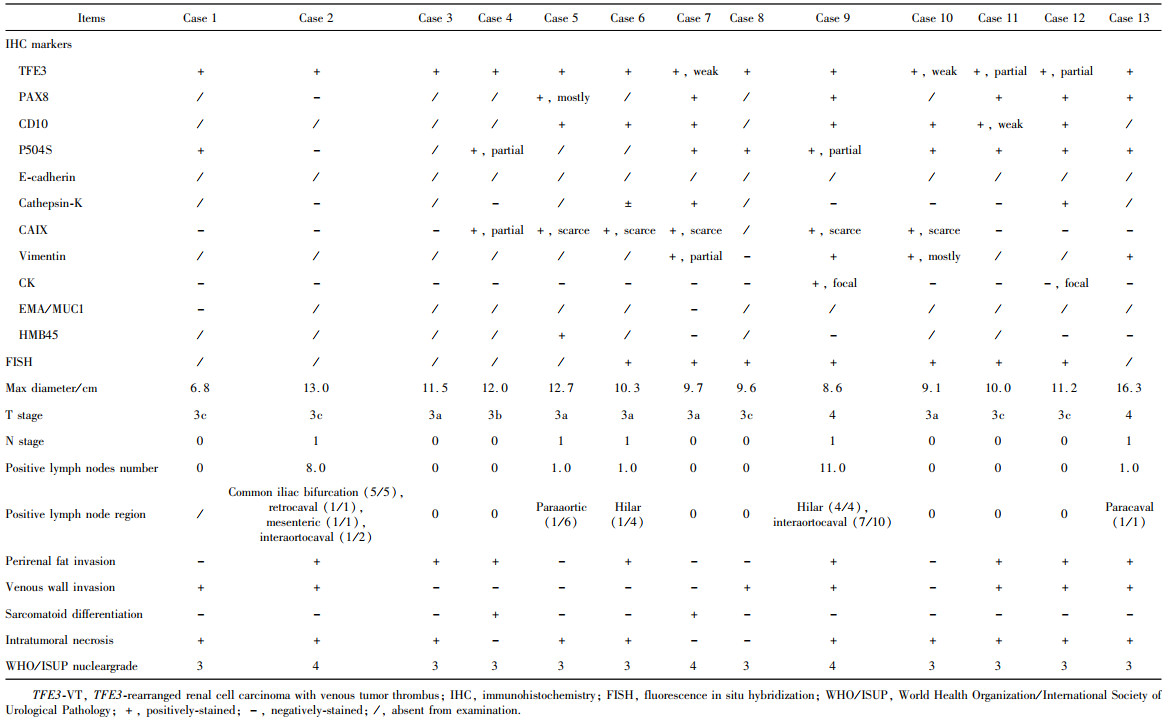 |
图2 1例合并Mayo Ⅱ级下腔静脉癌栓的TFE3重排肾细胞癌患者(病例9)的病理图像Figure 2 Pathological images from a left-sided TFE3-rearranged renal cell carcinoma patient with Mayo level Ⅱ inferior vena cava thrombus (case 9) A, Hematoxylin-Eosin staining (×200), tumor cells exhibit clear cytoplasm arranged in a sheet-like pattern; B, immunohistochemistry staining of PAX-8 (×200), staining of tumor cell nuclei was strongly positive for PAX-8; C, immunohistochemistry staining of TFE3 protein (×200), staining of tumor cell nuclei was strongly positive for TFE3, FISH-TFE3 showed red-green split signals (inset). |
2.3 生存分析
2.3.1 13例TFE3 -VT患者的生存随访情况
表4 13例TFE3 -VT患者的术后治疗及生存结局Table 4 Postoperative therapy and survival outcomes of 13 TFE3 -VT patients |
| Items | Case 1 | Case 2 | Case 3 | Case 4 | Case 5 | Case 6 | Case 7 |
| Postoperative therapy | TKI | TKI | TKI | None | TKI | TKI | TKI, ICI |
| Type of TKI | Sunitinib, axitinib | Sunitinib | Sunitinib | None | Pazopanib | NA | Sunitinib, axitinib, anlotinib |
| Type of ICI | None | None | None | None | None | None | Triprolizumab, bevacizumab (peritoneal perfusion) |
| Death | + | + | + | + | + | - | + |
| Death reason | Cancer | NA | Cancer | Cancer | Cancer | None | Cancer |
| OS/months | 56 | 3 | 9 | 12 | 8 | 16 | 13 |
| Progression | + | + | + | + | + | + | + |
| Sites of tumor relapse or metastasis | Lung, liver | NA | Abdominal wall, surgical region | Lung, liver | Liver | Lung, liver | Liver, peritoneum, omentum, retroperitoneal region |
| PFS/months | 13 | 3 | 4 | 3 | 1 | 11 | 6 |
| Items | Case 8 | Case 9 | Case 10 | Case 11 | Case 12 | Case 13 | |
| Postoperative therapy | TKI | TKI | TKI | TKI, radiotherapy, ICI | None | TKI, ICI | |
| Type of TKI | Sunitinib, axitinib | Pazopanib | Pazopanib | Sunitinib, axitinib | None | Axitinib | |
| Type of ICI | None | None | None | Pembrolizumab | None | Pembrolizumab | |
| Death | + | + | - | - | + | - | |
| Death reason | Cancer | Cancer | None | None | Cancer | None | |
| OS/months | 40 | 1 | 34 | 31 | 2 | 16 | |
| Progression | + | + | + | - | + | - | |
| Sites of tumor relapse or metastasis | Lung | Liver, adrenal gland, abdominal wall | Surgical region, cervical and mediastinal lymph nodes, lung | None | NA | None | |
| PFS/months | 1 | 0 | 13 | 31 | 2 | 16 | |
TFE3 -VT, TFE3 -rearranged renal cell carcinoma with venous tumor thrombus; TKI, tyrosine kinase inhibitor; ICI immune checkpoints inhibitor; OS, overall survival; PFS, progression free survival; NA, not available; +, presence of survival outcome; -, censoring at the latest follow-up. |
2.3.2 临床Ⅰ~Ⅳ期TFE3 -RCC患者生存情况比较
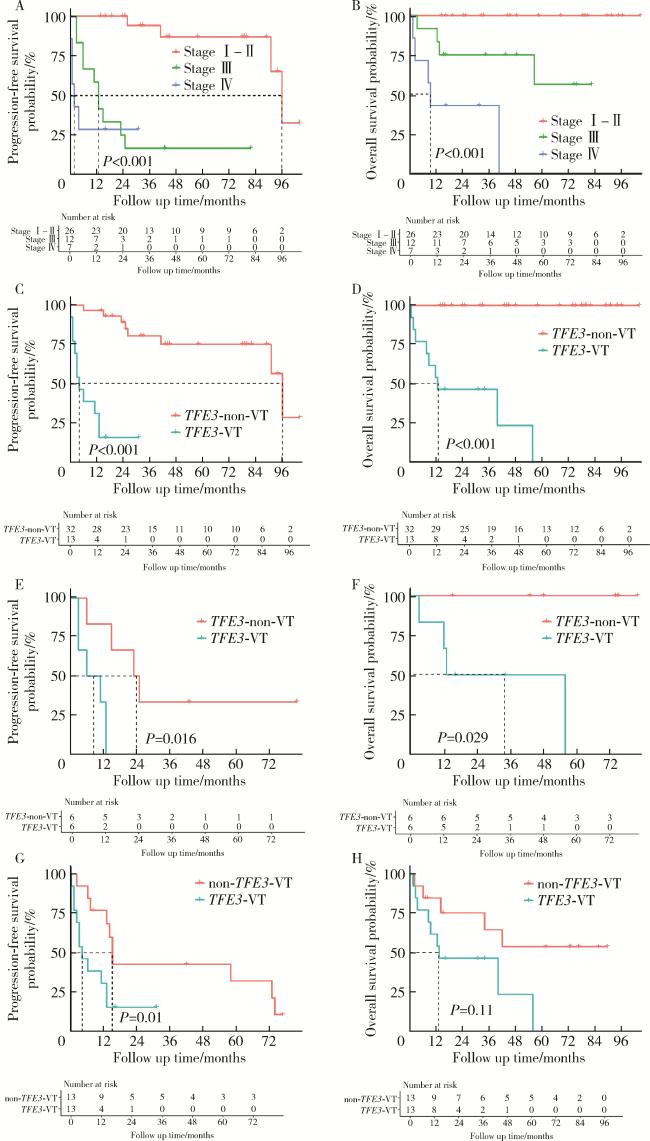
图3 TFE3重排肾细胞癌合并静脉癌栓患者、不合并静脉癌栓的TFE3重排肾细胞癌患者以及非TFE3突变的肾细胞癌合并静脉癌栓患者的生存曲线Figure 3 Kaplan-Meier curves of TFE3 -VT, TFE3 -non-VT, and non- TFE3 -VT A, B, progression free survival and overall survival curves of TFE3-RCC patients with different clinical tumor stage (2017 American Joint Committee on Cancer 8th version); C, D, progression free survival and overall survival curves of TFE3-VT and TFE3-non-VT patients; E, F, progression free survi-val and overall survival curves of stage Ⅲ TFE3 -VT and TFE3 -non-VT patients.; G, H, progression free survival and overall survival curves of TFE3 -VT and non-TFE3 -VT patients after 1 ∶ 1 propensity score matching. TFE3 -RCC, TFE3 -rearranged renal cell carcinoma; TFE3 -VT, TFE3 -rearranged renal cell carcinoma with venous tumor thrombus; TFE3 -non-VT, TFE3-rearranged renal cell carcinoma patients without VT; non-TFE3 -VT, non-TFE3 -rearranged renal cell carcinoma patients with VT. |
2.3.3 TFE3 -VT与TFE3 -non-VT患者的生存比较
2.3.4 TFE3 -VT与non- TFE3 -VT患者的生存比较
表5 倾向性评分匹配前后匹配因素的分布情况Table 5 Distribution of matching factors before and after propensity score matching (PSM) |
| Items | Before PSM | SMD | After PSM | SMD | ||
| TFE3-VT (n=13) | non-TFE3-VT (n=523) | TFE3-VT (n=13) | non-TFE3-VT (n=13) | |||
| Age/years, n (%) | 1.432 | < 0.001 | ||||
| ≤45 | 8 (61.5) | 60 (11.5) | 8 (61.5) | 8 (61.5) | ||
| >45-65 | 5 (38.5) | 312 (59.7) | 5 (38.5) | 5 (38.5) | ||
| >65 | 0 (0.0) | 151 (28.9) | 0 (0.0) | 0 (0.0) | ||
| Gender, n (%) | 0.857 | < 0.001 | ||||
| Male | 4 (30.8) | 367 (70.2) | 4 (30.8) | 4 (30.8) | ||
| Female | 9 (69.2) | 156 (29.8) | 9 (69.2) | 9 (69.2) | ||
| M1 stage, n (%) | 7 (53.8) | 144 (27.5) | 0.556 | 7 (53.8) | 7 (53.8) | < 0.001 |
| Neoadjuvant therapy, n (%) | 4 (30.8) | 66 (12.6) | 0.451 | 4 (30.8) | 4 (30.8) | < 0.001 |
PSM, propensity score matching; TFE3 -VT, TFE3 -rearranged renal cell carcinoma with venous tumor thrombus; non-TFE3-VT, non-TFE3-rearranged renal cell carcinoma patients with venous tumor thrombus; SMD, standard mean deviation. |