1 资料与方法
1.1 研究对象
1.2 仪器与试剂
1.3 标本采集方法
1.4 统计学方法
2 结果
2.1 血清炎症指标和细胞因子水平
表1 两组血清炎症指标和细胞因子水平Table 1 Serum inflammatory indexes and cytokines levels in both groups |
| Items | Healthy controls (n=38) | Chronic periodontitis (n=42) | P |
| CRP/(mg/L) | 0.60 (0.36, 1.62) | 1.49 (0.34, 4.78) | 0.035 |
| SAA/(mg/L) | 1.16 (0.92, 1.69) | 2.18 (1.73, 7.18) | < 0.001 |
| PCT/(ng/L) | 6.50 (2.75, 13.30) | 12.00 (3.00, 21.00) | 0.128 |
| IL-1β/(ng/L) | 1.04 (0.51, 1.65) | 0.50 (0.00, 2.52) | 0.285 |
| IL-2/(ng/L) | 1.42 (1.05, 2.16) | 2.46 (1.71, 3.75) | < 0.001 |
| IL-4/(ng/L) | 1.40 (0.75, 2.11) | 1.26 (0.67, 1.95) | 0.491 |
| IL-5/(ng/L) | 1.24 (0.73, 1.74) | 1.84 (0.91, 3.93) | 0.031 |
| IL-6/(ng/L) | 0.72 (0.45, 1.02) | 0.24 (0.00, 1.61) | 0.095 |
| IL-8/(ng/L) | 4.27 (3.31, 5.77) | 47.25 (28.59, 76.37) | < 0.001 |
| IL-10/(ng/L) | 1.44 (0.72, 1.98) | 1.51 (1.33, 1.98) | 0.116 |
| IL-12P70/(ng/L) | 0.99 (0.78, 1.73) | 1.99 (1.45, 2.35) | < 0.001 |
| IL-17/(ng/L) | 2.01 (1.36, 2.47) | 3.33(2.39, 4.44) | < 0.001 |
| IFN-α/(ng/L) | 1.53 (0.92, 2.61) | 2.42(1.53, 4.30) | 0.002 |
| IFN-γ/(ng/L) | 3.69 (2.56, 5.23) | 4.16 (2.80, 5.54) | 0.784 |
| TNF-α/(ng/L) | 2.15 (1.63, 2.59) | 1.49 (0.51, 3.17) | 0.158 |
Data are expressed as median (P25, P75). CRP, C-reactive protein; SAA, serum amyloid A; PCT, procalcitonin; IL, interleukin; IFN, inter-feron; TNF, tumor necrosis factor. |
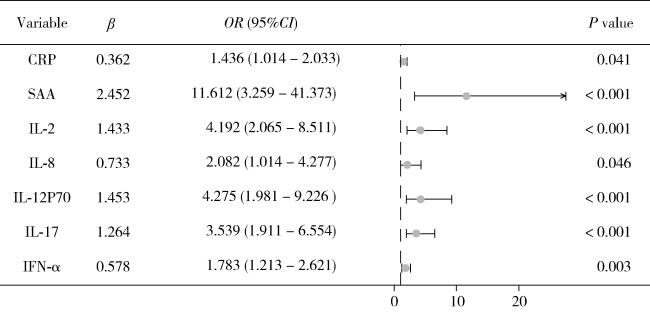
图1 二元Logistic回归筛选血清炎症指标和细胞因子的森林图Figure 1 Forest plot of serum inflammatory indexes and cytokines screened by binary Logistic regression CRP, C-reactive protein; SAA, serum amyloid A; IL, interleukin; IFN, interferon. |
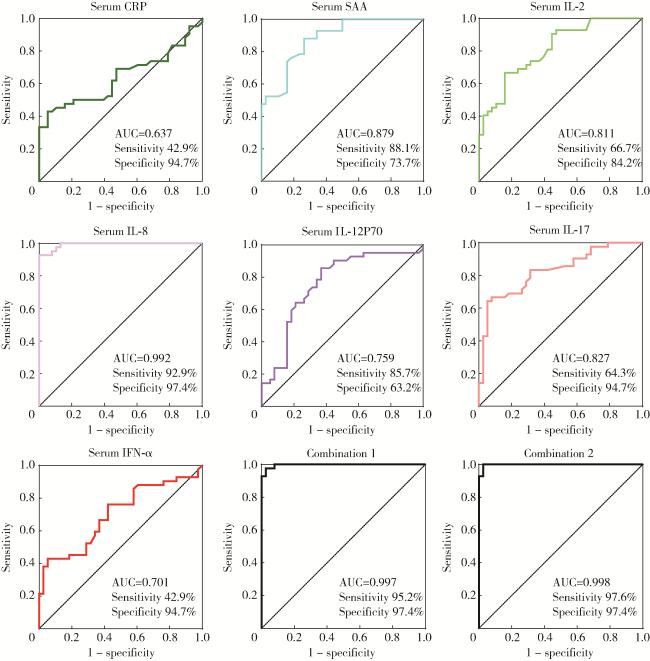
图2 受试者工作特征曲线评估血清炎症指标和细胞因子的诊断效能Figure 2 Receiver operating characteristic curve evaluated the diagnostic efficacy of serum inflammatory indexes and cytokines CRP, C-reactive protein; SAA, serum amyloid A; IL, interleukin; IFN, interferon; AUC, area under curve. Combination 1, IL-2+IL-8; Combination 2, CRP+SAA+IL-12P70. |
2.2 唾液细胞因子水平
表2 两组唾液细胞因子水平Table 2 Saliva cytokines levels in both groups |
| Items | Healthy controls (n=38) | Chronic periodontitis (n=42) | P |
| IL-1β/(ng/L) | 231.53 (124.49, 587.13) | 427.51 (171.01, 805.16) | 0.025 |
| IL-2/(ng/L) | 2.42 (1.65, 3.49) | 2.71 (2.19, 3.49) | 0.075 |
| IL-4/(ng/L) | 1.35 (0.74, 1.86) | 0.98 (0.70, 1.22) | 0.019 |
| IL-5/(ng/L) | 2.42 (0.97, 7.15) | 1.87 (1.26, 2.41) | 0.336 |
| IL-6/(ng/L) | 16.46 (7.85, 31.68) | 41.55 (20.84, 55.25) | 0.002 |
| IL-8/(ng/L) | 343.02 (253.26, 798.46) | 932.71 (671.42, 1 467.16) | < 0.001 |
| IL-10/(ng/L) | 2.54 (1.65, 4.25) | 1.92 (1.51, 3.29) | 0.401 |
| IL-12P70/(ng/L) | 1.77 (1.38, 2.84) | 2.18 (1.87, 2.72) | 0.032 |
| IL-17/(ng/L) | 2.92 (1.92, 4.73) | 2.57 (1.68, 4.75) | 0.919 |
| IFN-α/(ng/L) | 2.04 (1.53, 2.81) | 2.36 (1.25, 3.03) | 0.612 |
| IFN-γ/(ng/L) | 2.69 (1.62, 5.47) | 2.42 (1.44, 5.02) | 0.771 |
| TNF-α/(ng/L) | 6.95 (4.37, 9.18) | 4.74 (1.38, 7.93) | 0.066 |
Data are expressed as median (P25, P75). IL, interleukin; IFN, interferon; TNF, tumor necrosis factor. |
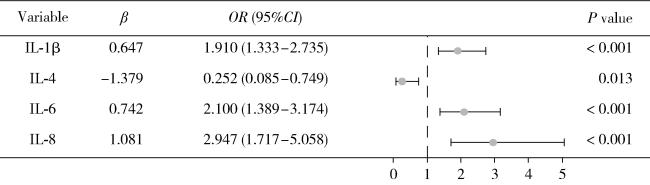
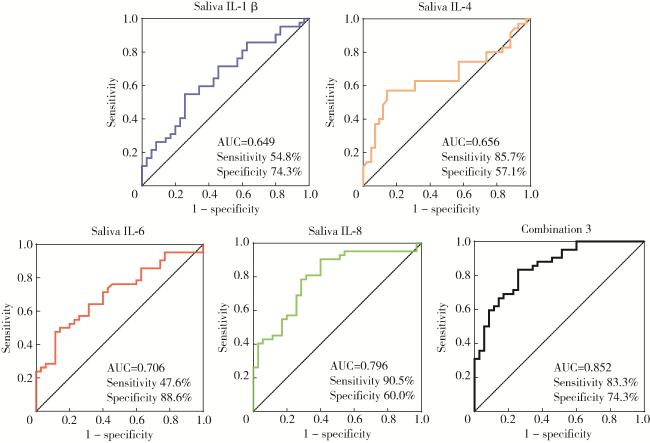
图3 二元Logistic回归筛选唾液细胞因子的森林图Figure 3 Forest plot of saliva cytokines screened by binary Logistic regression IL, interleukin. |
2.3 炎症指标和细胞因子水平与慢性牙周炎分期的相关性分析
表3 炎症指标和细胞因子水平与慢性牙周炎严重程度的相关性Table 3 Correlation of inflammatory indexes and cytokines with severity of chronic periodontitis |
| Index | ρ | P |
| Serum | ||
| CRP | 0.450 | 0.003 |
| SAA | 0.368 | 0.017 |
| IL-8 | 0.460 | 0.002 |
| Saliva | ||
| IL-1β | 0.523 | < 0.001 |
| IL-6 | 0.470 | 0.002 |
| IL-8 | 0.732 | < 0.001 |
| IL-12P70 | 0.484 | 0.001 |
| IL-17 | 0.439 | 0.004 |
| TNF-α | 0.334 | 0.030 |
CRP, C-reactive protein; SAA, serum amyloid A; IL, interleukin; TNF, tumor necrosis factor. |