1 血浆基质的分类及作用机制
1.1 血浆基质的分类
1.2 血浆基质促进再生的机制
1.3 血浆基质在口腔种植中的应用形式
2 血浆基质在口腔种植相关领域的临床应用
2.1 引导骨组织再生术
2.2 牙槽嵴保存术
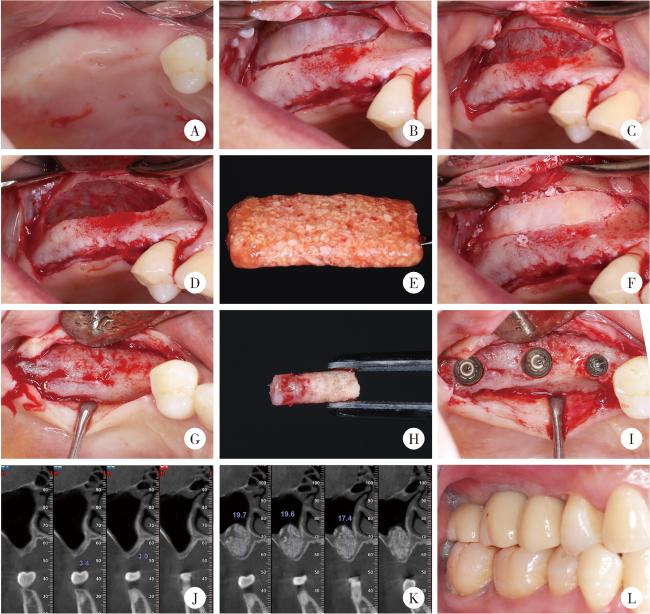
2.3 上颌窦底提升术
图1 血浆基质骨块在上颌窦底提升术中的临床应用示例Figure 1 Clinical application of a representative plasmatrix bone block in maxillary sinus floor elevation A, intraoral view before surgery; B, preparation of the lateral window; C, exposure of the Schneiderian membrane; D, elevation of the Schneiderian membrane; E, plasmatrix bone block; F, repositioning of the lateral window after grafting with the bone block; G, alveolar bone condition six months after sinus floor elevation; H, intact bone column at the implant site; I, implant placement; J, preoperative CBCT showing insufficient alveolar bone height; K, CBCT at six months postoperatively showing adequate bone height; L, occlusion after prosthetic restoration. CBCT, cone beam CT. |