1 资料与方法
1.1 研究对象
1.2 主要试剂及仪器
1.3 血清LUM的测定
1.4 临床数据的采集
1.5 疾病活动度评估
1.6 统计学分析
2 结果
2.1 研究对象基本资料
表1 RA患者一般人口学、临床特征和血清LUM水平Table 1 General demography, clinical characteristics and serum LUM level in patients with RA |
| Characteristics | Data |
| RA (n=133) | |
| Seropositive | 115 (86.5) |
| Seronegative | 18 (13.5) |
| Gender (n=133) | |
| Male | 32 (24.1) |
| Female | 101 (75.9) |
| Age/years | 58.26±14.31 |
| Laboratory features | |
| ESR/(mm/h) (n=124) | 36.00 (13.25, 73.00) |
| CRP/(mg/L) (n=131) | 12.52 (2.80, 50.29) |
| RF/(IU/mL) (n=133) | 86.60 (23.05, 280.00) |
| Anti-CCP/(U/mL) (n=126) | 234.95 (73.58, 444.73) |
| IgA/(g/L) (n=105) | 2.83 (1.97, 3.90) |
| IgM/(g/L) (n=105) | 1.26 (0.91, 1.59) |
| IgG/(g/L) (n=105) | 13.73 (11.36, 17.00) |
| C3/(g/L) (n=104) | 1.22±0.31 |
| C4/(g/L) (n=104) | 0.20 (0.16, 0.26) |
| PLT/(×109/L) (n=125) | 268 (203, 339) |
| RA activity (score according to DAS28-CRP) (n=133)* | |
| HDA (n=64) | 23.66±5.13 |
| MDA (n=35) | 22.37±5.13 |
| LDA (n=8) | 21.92±6.15 |
| Remission (n=20) | 18.88±5.19 |
| Complications (n=133) | |
| ILD | 16 (12.0) |
| SS | 10 (7.5) |
| TGD | 38 (28.6) |
| OP | 50 (37.6) |
| LUM/(μg/L) (n=133) | 22.17±5.47 |
Data are presented as $\bar x \pm s$, n (%) or M(P25, P75). *, the amount of missing data was 6. RA, rheumatoid arthritis; LUM, lumican; ESR, erythrocyte sedimentation rate; CRP, C-reactive protein; RF, rheumatoid factor; Anti-CCP, anti-cyclic citrullinated peptide; IgA, immunoglobulin A; IgG, immunoglobulin G; IgM, immunoglobulin M; C3, complement 3; C4, complement 4; PLT, platelet counts; DAS28, 28-joint disease activity score; HDA, high disease activity; MDA, middle disease activity; LDA, low disease activity; ILD, interstitial lung disease; SS, Sjögren syndrome; TGD, thyroid gland diseases; OP, osteoporosis. |
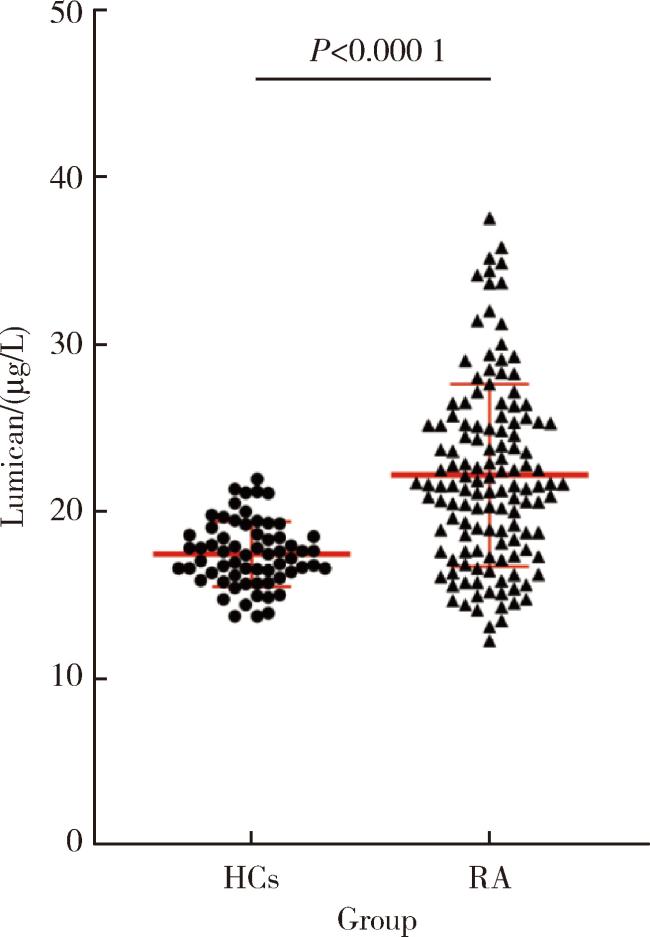
2.2 RA患者和健康对照组血清中LUM表达水平的比较
2.3 LUM与临床指标的相关性分析
表2 RA患者血清LUM水平与临床指标的相关性Table 2 Correlation between serum LUM level and clinical characteristics in patients with RA |
| Characteristics | Serum LUM levels | |
| R | P | |
| Age | 0.132 | 0.130 |
| Disease duration | 0.032 | 0.715 |
| ESR | 0.295 | 0.001** |
| CRP | 0.229 | 0.008** |
| RF | 0.366 | <0.001*** |
| Anti-CCP | 0.129 | 0.150 |
| IgA | 0.227 | 0.020** |
| IgM | 0.163 | 0.097 |
| IgG | 0.170 | 0.082 |
| C3 | 0.128 | 0.195 |
| C4 | -0.147 | 0.136 |
| PLT | 0.299 | 0.001** |
| DAS28-CRP | 0.274 | 0.002** |
**P<0.01, ***P<0.001, with exact P values shown in the table (two-tailed Spearman’s rank correlation test). Abbreviations as in Table 1. |
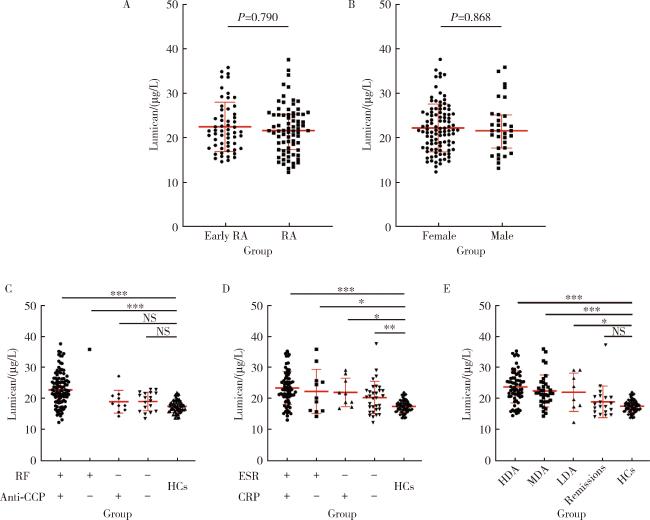
2.4 不同特征RA患者血清LUM表达的差异
图2 不同临床及实验室特征的RA患者血清LUM水平Figure 2 Serum LUM levels in RA patients with different clinical and laboratory characteristics *P < 0.05, **P < 0.01, ***P < 0.001. A, serum LUM levels in RA patients with different disease durations (early RA: ≤ 2 years; RA: > 2 years); B, serum LUM levels in RA patients with different sexes; C, serum LUM levels in RA patients with different levels of RF and anti-CCP; D, serum LUM levels in RA patients with different levels of CRP and ESR; E, RA patients with different disease activity (Remission: DAS28 ≤ 2.6; LDA: 2.6 < DAS28 ≤ 3.2; MDA: 3.2 < DAS28 ≤ 5.1; HDA: DAS28 > 5.1). HCs, healthy controls; NS, no significant; Other abbreviations as in Table 1. |
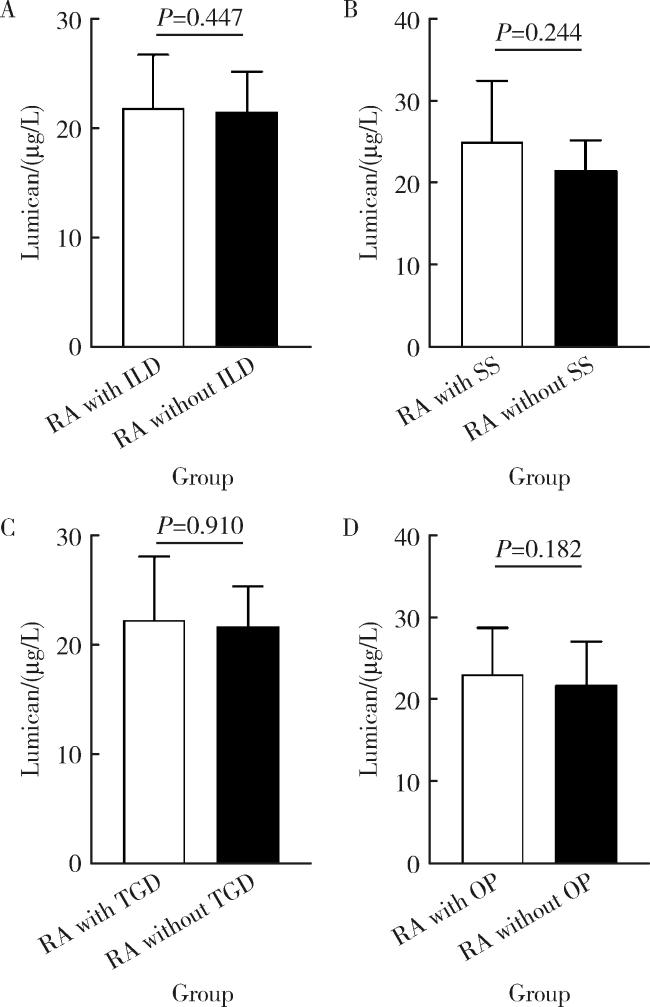
2.5 不同合并症RA患者血清LUM的表达差异
图3 合并或不合并各种并发症的RA患者血清LUM水平Figure 3 Serum LUM levels in RA patients with or without different complications or comorbidities A, RA patients with or without interstitial lung disease (ILD); B, RA patients with or without Sjögren syndrome (SS); C, RA patients with or without thyroid gland diseases (TGD); D, RA patients with or without osteoporosis (OP). RA, rheumatoid arthritis; LUM, lumican. |
2.6 不同血清RF、抗CCP抗体水平的RA患者血清LUM阳性率
表3 不同RF和抗CCP抗体水平的RA患者血清LUM阳性率Table 3 The positive rate of LUM in RA patients with different levels of serum RF or anti-CCP |
| Group | RA with LUM, n (%) | RA without LUM, n (%) | χ2 value | P value |
| RF+ | 63 (60.0) | 42 (40.0) | 8.774 | 0.003 |
| RF- | 8 (28.6) | 20 (71.4) | ||
| Anti-CCP+ | 64 (59.8) | 43 (40.2) | 3.461 | 0.063 |
| Anti-CCP- | 7 (36.8) | 12 (63.2) |
Abbreviations as in Table 1. |