目的:探讨位于雄激素受体(androgen receptor, AR)配体结合域中具有出核转运信号肽的核转出蛋白(nuclear export signal of androgen receptor, NESAR)对前列腺癌雄激素受体蛋白表达和稳定性调节的机制。方法:绿色荧光蛋白(green fluorescent protein, GFP)融合蛋白表达载体pEGFP-AR(1-918aa),pEGFP-NESAR(743-817aa),pEGFP-NAR (1-665aa)和pEGFP-NAR-NESAR,及NESAR的赖氨酸突变体(赖氨酸K突变为精氨酸R)pEGFP-NESAR K776R,pEGFP-NESAR K807R和pEGFP-NESAR K776R/K807R,瞬时转染前列腺癌细胞PC3后,采用荧光显微镜、蛋白质免疫印迹和免疫沉淀,检测NESAR对雄激素受体稳定性的调节。结果:在荧光显微镜下,含NESAR的融合蛋白呈胞浆定位,荧光信号强度比不含NESAR的融合蛋白明显减弱,且含NESAR的融合蛋白表达水平显著低于不含NESAR的融合蛋白表达水平。在蛋白合成抑制剂放线菌酮处理下,GFP-NESAR和GFP-NAR-NESAR的半衰期小于6 h,而对照GFP和GFP-NAR的表达相对更稳定,半衰期大于24 h。融合蛋白GFP-NESAR在蛋白酶体抑制剂MG132的处理下,其蛋白表达显著增加,并呈现剂量依赖性,而MG132对GFP蛋白稳定性没有显著影响;在蛋白合成被抑制的情况下,MG132同样显著抑制了GFP-NESAR融合蛋白的降解,且蛋白表达呈剂量依赖性的增加。GFP免疫沉淀的结果显示,GFP-NESAR融合蛋白的泛素化水平明显高于GFP对照。赖氨酸位点K776和K807突变的NESAR的泛素化水平显著降低,且蛋白稳定性提高,赖氨酸位点K776和K807是介导NESAR发生泛素化的关键氨基酸残基。结论:核转出蛋白NESAR具有降解不稳定性,作为多聚泛素化修饰的识别信号,介导了其融合蛋白在前列腺癌细胞中通过泛素-蛋白酶体途径的降解。本研究为AR蛋白水平和/或活性的调控开辟一种新的研究思路,有助于对AR降解分子机制的了解和在前列腺癌产生去势抗性的过程中对AR靶向的密切调控。
Objective:To investigate the mechanisms of nuclear export signal of androgen receptor (NESAR) in the regulation of androgen receptor (AR) protein expression and stability in prostate cancer.Methods:The green fluorescent protein fusion protein expression vectors pEGFP-AR(1-918aa), pEGFP-NESAR (743-817aa), pEGFP-NAR (1-665aa) and pEGFP-NAR-NESAR, and lysine mutants of NESARpEGFP-NESAR K776R, pEGFP-NESAR K807R and pEGFP-NESAR K776R/K807R, were transiently transfec-ted into prostate cancer cell line PC3. Fluorescence microscopy, Western blot and immunoprecipitation were used to detect NESAR regulation of androgen receptor stability.Results:Under the fluorescence microscope, NESAR-containing fusion proteins were cytoplasmic localization, and their fluorescence intensities were much weaker than those without NESAR. The expression levels of NESAR-containing fusion proteins were significantly lower than those without NESAR. The half-lives of GFP-NESAR and GFP-NAR-NESAR were less than 6 h, while the expression of GFP and GFP-NAR was relatively stable and the half-life was more than 24 h in the presence of cycloheximide. The expression levels of GFP-NESAR were significantly increased by proteasome inhibitor MG132 treatment in a dose-dependent manner; in contrast, MG132 did not show any significant effect on the protein levels of GFP. When new protein synthesis was blocked, MG132 could also prevent the degradation of GFP-NESAR in the transfected cells in the presence of cycloheximide, while it had no significant effect on GFP protein stability in the parallel experiment. GFP immunoprecipitation showed that the ubiquitination level of GFP-NESAR fusion protein was significantly higher than that of the GFP control. The mutations of lysine sites K776 and K807 in NESAR significantly reduced the level of ubiquitination, and showed increased protein stability, indicating that they were the key amino acid residues of NESAR ubiquitination.Conclusion:NESAR was unstable and decreased the stability of its fusion proteins. NESAR was the target of polyubiquitination and mediated the degradation of its fusion proteins through the ubiquitin-proteasome pathway in prostate cancer cells. Our research provides a new way to regulate the level and/or activity of AR proteins, thus helping us understand the molecular mechanisms of AR degradation and strict control of AR in the progression to castration-resistance.
前列腺癌是最常见的男性恶性肿瘤之一, 在我国的发病率虽然远低于西方国家, 但随着生活水平和诊断技术的不断提高, 其发病率呈上升趋势。前列腺癌细胞的生长最初依赖于循环的雄激素, 此时的雄激素剥夺疗法可以有效地抑制肿瘤细胞的生长; 但不断的雄激素剥夺最终导致细胞亚群的选择, 抗雄激素治疗后仍复发并进展为去势抵抗性前列腺癌(castration resistant prostate cancer, CRPC), 并继续增殖生长, 进而增加血管生成, 通过骨和淋巴结进行远处转移[1]。在雄激素依赖性和去势抵抗性前列腺癌中, 雄激素受体(androgen receptor, AR)均具有转录活性, 且在CRPC进展过程中发挥着重要的作用[2]。AR的胞浆/胞核穿梭是AR活性调控中的重要一步, AR进核之后作为转录因子发挥其功能, AR的异常核定位常与前列腺癌产生去势抵抗性密切相关[3]。调控AR活性的另一机制是对AR蛋白稳定性的调控, AR表达和/或稳定性的增加, 可以使前列腺癌细胞对低浓度雄激素的敏感性增高[4], 是CRPC产生的重要机制之一, 因此, 了解调控AR稳定性和/或降解的具体机制, 将具有重要的临床参考和应用价值, 尤其是在前列腺癌由雄激素依赖性进展为去势抵抗性的过程中。
细胞内80%~90%的蛋白质和大多数的细胞外蛋白质都在不断地被降解, 并被新合成的蛋白质取代。细胞外的蛋白质降解主要是通过蛋白酶的作用, 而细胞内蛋白质的降解可以分为两类, 一是由溶酶体介导的自噬, 另一种是由泛素-蛋白酶体介导的蛋白泛素化降解。泛素-蛋白酶体系统(ubiquitin proteasome system, UPS)是由泛素介导的一种高度复杂的蛋白降解机制, 它参与降解细胞内许多蛋白质并且这个过程具有高度特异性。泛素-蛋白酶体途径通过对特殊蛋白质的降解, 在真核细胞的分化、增殖、凋亡、基因转录、信号转导、新陈代谢调节、免疫监视等生命活动中起至关重要的作用[5]。泛素-蛋白酶体系统与疾病的发生发展密切相关, 如肿瘤、病毒感染、神经变性疾病、代谢性疾病及炎症等[6, 7, 8]。由于泛素化系统在细胞活动中的重要作用, 认识细胞内蛋白泛素化降解作用机制, 对认识由泛素系统失调引起的各种疾病尤其是恶性肿瘤具有重要意义。
具有出核转运信号肽的核转出蛋白(nuclear export signal of androgen receptor, NESAR)是位于雄激素受体配体结合域中的一段蛋白序列, 即743-817aa。前期工作中发现, NESAR在AR的细胞浆/细胞核穿梭中具有重要作用, 在配体雄激素缺乏情况下, NESAR占主导并指导AR的出核; 在配体雄激素存在的情况下, NESAR的作用被抑制, AR表现为核定位[9]; 同时我们还发现NESAR的蛋白表达具有不稳定性。本研究在前期研究的基础上, 分析明确NESAR的降解不稳定性, 探讨其调控雄激素受体AR的表达及稳定性的机制, 从而有助于我们对AR降解分子机制的了解和在前列腺癌产生去势抗性的过程中对AR靶向的密切调控。
本研究所用人前列腺癌细胞株PC3购自美国ATCC公司。绿色荧光蛋白(green fluorescent protein, GFP)融合蛋白表达载体pEGFP-AR(1-918aa), pEGFP-NESAR(743-817aa), pEGFP-NAR (1-665aa)和pEGFP-NAR-NESAR均为美国匹兹堡大学泌尿外科研究中心王洲实验室构建并惠赠。NESAR的赖氨酸突变体(赖氨酸K突变为精氨酸R)pEGFP-NESAR K776R, pEGFP-NESAR K807R和pEGFP-NESAR K776R/K807R的构建, 参见StarMut 基因定点突变试剂盒说明书, 购自北京康润诚业生物科技有限公司。
RPMI1640(roswell park memorial institute 1640)细胞培养液、胎牛血清(fetal bovine serum, FBS)、100 U/mL青霉素和100 mg/L链霉素购自美国GIBCO公司。蛋白合成抑制剂放线菌酮(cycloheximide, CHX)和蛋白酶体抑制剂MG132均购自美国Sigma-Aldrich公司。PolyJetTM 体外DNA转染试剂盒购自美国SignaGen公司。GFP抗体购自德国Acris, 泛素蛋白(ubiquitin, Ub)抗体(sc8017)、内参照GAPDH抗体(sc-25778)和辣根过氧化物酶标记的山羊抗鼠/兔 IgG (sc-2004/2005)均购于美国Santa Cruz Biotecnology公司。抗GFP标签的单克隆抗体偶联的琼脂糖凝胶珠(agarose conjugated anti-GFP-tag)购自北京博尔迈生物技术有限公司。二喹啉甲酸(bicinchoninic acid, BCA)蛋白定量试剂盒和增强化学发光(enhanced chemiluminescence, ECL)试剂盒购自美国Thermo公司。
人前列腺癌细胞株PC3用含有10%(体积分数)FBS、100 U/ml青霉素和100 mg/L链霉素的RPMI1640培养液培养于37 ℃、5% (体积分数)CO2饱和湿度的培养箱内, 当细胞生长融合成单层时, 用胰蛋白酶消化并传代培养。在细胞转染的前一天, 在每个10 cm的培养皿中接种适宜数量的细胞, 使得在转染的时候达到80%~85%的密度; 转染时更换成新鲜的完全培养液; 根据PolyJetTM 体外DNA转染试剂盒说明进行质粒转染, 转染12~18 h后更换为新鲜的完全培养液; 加入50 mg/L CHX和/或指定浓度的MG132处理指定时间后收集细胞。
按指定时间处理细胞后, 弃去培养液, 用预冷磷酸盐缓冲液(phosphate buffered saline, PBS)清洗两次, 加入一定量改良型的放射免疫沉淀蛋白裂解缓冲液(radioimmune precipitation assay buffer, RIPA)提取总蛋白, 裂解液组成:50 mmol/L Tris (pH 7.4), 1% (体积分数)乙基苯基聚乙二醇(Nonidet P-40), 0.25%(质量分数)脱氧胆酸钠(Na-deoxycholate), 150 mmol/L NaCl, 1 mmol/L EDTA (pH 8.0), 1 mmol/L NaF, 2 mmol/L苯甲基磺酰氟(phenylmethylsulfonyl fluoride, PMSF), 1 mmol/L钒酸钠(Na3VO4)和蛋白酶抑制剂。采用BCA法测定蛋白浓度; 加入5× 上样缓冲液, 100 ℃煮沸10 min。取等量蛋白进行8%~12%(质量分数) 十二烷基硫酸钠聚丙烯酰胺凝胶电泳(sodium dodecyl sulfate polyacrylamide gel electrophoresis, SDS-PAGE)分离蛋白质, 300 mA转膜70 min, 5%(质量分数) 脱脂奶粉室温封闭1 h, 一抗4 ℃孵育过夜, 三羟甲基氨基甲烷缓冲盐水(triethanolamine buffered saline solution-Tween, TBST)洗膜3次× 10 min, 二抗室温孵育1 h, TBST洗膜3次× 10 min; 暗室加入ECL发光液后曝光显影。
对于免疫沉淀, 取一定体积的GFP标记的琼脂糖凝珠, 加入500 μ L预冷细胞裂解液清洗两次, 5 000 r/min 4 ℃离心30 s, 吸除上清液; 加入含等量蛋白的细胞裂解液, 补足细胞裂解液致体积达1 500 μ L, 再4 ℃旋转摇动过夜; 5 000 r/min 4 ℃离心1 min, 吸除上清液; 加入500 μ L冷细胞裂解液, 重复清洗琼脂糖凝珠3次, 4 ℃ 15 min/次; 加入上样缓冲液, 沸水浴中10 min使蛋白质变性, 然后进行蛋白质免疫印记检测分析。
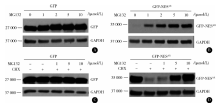
雄激素受体的配体结合域中具有出核转运信号肽的核转出蛋白NESAR(图1A), 在雄激素受体的细胞浆/细胞核穿梭中具有重要作用。在配体雄激素缺乏的情况下, NESAR占主导并指导AR的出核; 在配体雄激素存在的情况下, NESAR的作用被抑制, AR表现为核定位[9]。同时, 我们发现将融合蛋白表达载体pEGFP, pEGFP-NESAR(743-817aa), pEGFP-NAR (1-665aa)和pEGFP-NAR-NESAR瞬时转染雄激素非依赖性前列腺癌PC3细胞后, 在荧光显微镜下观察到, 含NESAR的融合蛋白(GFP-NESAR和GFP-NAR-NESAR)呈胞浆定位, 且荧光信号强度比不含NESAR的融合蛋白(GFP和GFP-NAR)明显减弱(图1B); 进一步的结果显示, 含NESAR融合蛋白的表达水平比不含NESAR的融合蛋白低(图1C)。上述发现提示, NESAR除了作为出核信号发挥功能外, 在AR蛋白质的降解中也具有潜在的功能。为了确定NESAR对蛋白稳定性的影响, 我们用蛋白合成抑制剂CHX进行处理, 结果显示含NESAR的融合蛋白GFP-NESAR和GFP-NAR-NESAR具有降解不稳定性, 6 h 内其蛋白发生降解; 而不含NESAR的GFP-NAR和 GFP, 24 h内其蛋白表达相对稳定(图2)。以上结果提示, NESAR在前列腺癌细胞中作为一个新的降解基团, 在调节蛋白稳定性中发挥了重要的作用。
在一定浓度的蛋白酶体抑制剂MG132处理24 h 的情况下, 分析融合蛋白的表达变化, 发现MG132对GFP蛋白表达没有显著影响, 而含NESAR的融合蛋白GFP-NESAR在MG132的处理下, 其蛋白表达显著增加, 并呈现剂量依赖性(图3A、B)。进一步检测在蛋白合成被抑制的情况下, MG132是否可以抑制含NESAR的融合蛋白的降解, 如图3C、D结果显示, 在蛋白合成被抑制的情况下, MG132显著抑制了GFP-NESAR融合蛋白的降解, 蛋白表达呈剂量依赖性的增加; 而在对照实验中, MG132对GFP蛋白稳定性没有显著影响, 结果提示, NESAR通过蛋白酶体依赖的蛋白降解途径调节了融合蛋白的降解。
在泛素-蛋白酶体途径中, 蛋白酶体通常特异性地降解泛素化的蛋白。前面实验已证实, 蛋白酶体的抑制可以阻止NESAR融合蛋白的降解, 那么融合蛋白是否先发生了泛素化?我们在前列腺癌细胞系PC3中, 瞬时共转染带有His 标记的泛素(His-Ub)和GFP或GFP-NESAR, 然后用DMSO或蛋白酶体抑制剂MG132处理18~20 h。GFP免疫沉淀的结果显示, GFP-NESAR融合蛋白的泛素化水平明显高于GFP对照(图4), 表明NESAR作为泛素化识别信号, 引起融合蛋白的泛素化, 进而被蛋白酶体识别降解。
泛素蛋白是一个非常保守的多肽, 在泛素激活酶(E1)、泛素融合酶(E2)、泛素连接酶(E3)等一系列酶促反应催化下与细胞内靶蛋白上的一个或多个赖氨酸残基发生共价连接, 进行泛素化修饰。通过分析NESAR的蛋白质序列, 我们发现其存在两个赖氨酸残基(K776和K807), 进而构建了NESAR的赖氨酸突变体(赖氨酸K突变为精氨酸R), 以期确定NESAR序列中赖氨酸残基的突变是否会影响其泛素化修饰, 结果显示, 赖氨酸突变的NESAR的泛素化水平显著降低, 且蛋白稳定性提高(图5), 因此, NESAR蛋白序列中赖氨酸位点K776和K807是介导其发生泛素化的关键氨基酸残基。
了解前列腺癌细胞中AR调控的机制具有重要的临床意义, 而前列腺癌产生去势抗性常常与AR蛋白水平的增加密切相关[10]。除了转录水平的调控, AR蛋白降解/稳定性的调控也是重要的一方面[11]。已有研究表明, 泛素-蛋白酶体降解途径参与调控了AR的蛋白降解和稳定性, 如位于AR铰链区的富含脯氨酸、谷氨酰胺、丝氨酸和苏氦酸的约10个氨基酸组成的结构序列(proline-, glutamate-, serine-, and threonine-rich sequence, PEST)是UPS的降解识别信号, 移除PEST序列会延长AR的半衰期[12]。靶蛋白AR只有被E3连接酶识别后, 才会通过UPS途径降解; 研究表明, 泛素蛋白连接酶(murine double minute 2, Mdm2)在与AR的N端转录激活域和配体结合域结合后, 介导了AR的泛素化蛋白酶体途径降解[13]。蛋白酶体的抑制将抑制AR的转录激活、核定位和其与共调节子之间的相互作用, 而蛋白酶体亚基则可以呈剂量依赖性地提高AR的转录激活[14]。近年来, 泛素-蛋白酶体途径对AR调控的重要性, 以及它在前列腺癌发生发展过程中的重要作用, 使其成为前列腺癌潜在的治疗靶点之一[15, 16, 17, 18]。
本研究中, 我们证实了NESAR是AR泛素-蛋白酶体降解的另一识别信号, 并为雄激素受体蛋白稳定性的调节提供了新的思路。我们的实验表明, NESAR作为一种新的泛素化和蛋白酶体降解识别信号, 调节蛋白的稳定性; NESAR在诱导蛋白降解方面具有潜在的研究价值。含NESAR融合蛋白的表达水平明显低于不含NESAR的融合蛋白; CHX抑制蛋白重新合成的情况下, GFP-NESAR和GFP-NAR-NESAR的半衰期小于6 h, 而对照GFP和GFP-NAR的表达相对更稳定, 半衰期大于24 h。由此可见, NESAR的存在显著降低了融合蛋白的稳定性, 推测其在调控AR稳定性中可能发挥了重要的作用。同时我们发现NESAR蛋白序列中的赖氨酸位点K776和K807是介导其发生泛素化的关键氨基酸残基。
现有研究表明, 去势抵抗性前列腺癌细胞仍可以合成雄激素, 由此, NESAR介导的AR降解或将会被抑制。目前抑制前列腺癌细胞中雄激素合成的方法之一是使用阿比特龙(abiraterone), 以阻断雄激素合成过程中的关键步骤[19, 20]。阿比特龙能有效抑制去势抵抗性前列腺癌患者的睾酮水平, 从而有利于AR的降解。当然, NESAR介导的AR降解同时也可能受其他机制的调控, 比如AR的N端转录激活域和配体结合域之间的相互作用可能会影响NES的降解, 还有NES出核和/或降解过程中所涉及的细胞因子在前列腺癌进展过程中的改变, 也会影响AR的稳定性。那么, 这些细胞因子的识别和鉴定也将会为靶向去势抵抗性前列腺癌中AR的降解提供新的思路和方法。
本研究为雄激素受体AR蛋白水平的调控提供了新的研究思路, 同时揭示了AR的配体结合域除了具有转录激活、进核、出核功能和受雄激素调控外, 还包含了受雄激素调控的降解基团NESAR。NESAR介导AR降解的诱导, 能够有效阻断雄激素敏感和产生去势抵抗性的前列腺癌细胞中AR的作用, 因此靶向NESAR介导的AR的降解或许是前列腺癌治疗的有效方式之一, 尤其在去势抵抗性的前列腺癌中。此外, 由于NESs结构和功能上的保守性, 我们对NESAR功能机制的深入研究将对其他类固醇受体的研究具有重要的参考价值。
The authors have declared that no competing interests exist.
| [1] |
|
| [2] |
|
| [3] |
|
| [4] |
|
| [5] |
|
| [6] |
|
| [7] |
|
| [8] |
|
| [9] |
|
| [10] |
|
| [11] |
|
| [12] |
|
| [13] |
|
| [14] |
|
| [15] |
|
| [16] |
|
| [17] |
|
| [18] |
|
| [19] |
|
| [20] |
|