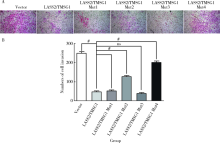
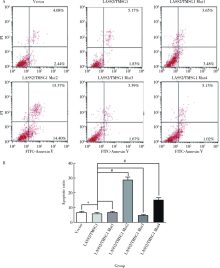
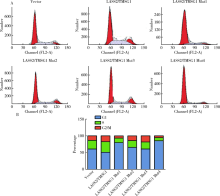
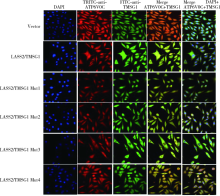
Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (2): 210-220. doi: 10.19723/j.issn.1671-167X.2019.02.003
Previous Articles Next Articles
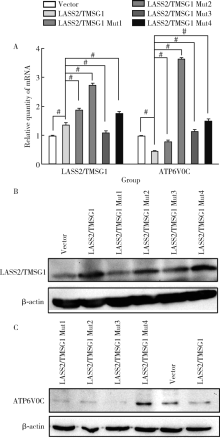
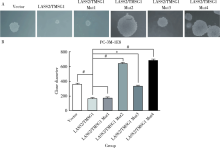
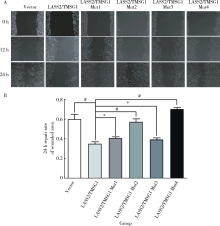
Novel tumor metastasis suppressorgene LASS2/TMSG1 S248A mutant promotes invasion of prostate cancer cells through increasing ATP6V0C expression
Kuan-gen ZHANG1,Yu-he ZHOU1,Ya-kun SHAO1,Fang MEI1,Jiang-feng YOU1,Bei-ying LIU2,Fei PEI1,3,∆( )
)
- 1. Department of Pathology, Peking University School of Basic Medical Sciences, Beijing 100191China
2. School of Mechanical Engineering, University of Science & Technology Beijing, Beijing 100083 China;
3. Department of Pathology, Peking University Third Hospital, Beijing 100191,China
CLC Number:
- R737.25
| [1] |
Cronin KA, Lake AJ, Scott S , et al. Annual Report to the nation on the status of cancer, part Ⅰ: national cancer statistics[J]. Cancer, 2018,124(13):2785-2800.
doi: 10.1002/cncr.31551 |
| [2] | 刘宇欣, 郑杰, 方伟岗 , 等. 具有不同转移潜能的前列腺癌细胞亚系的分离鉴定[J]. 中华病理学杂志, 1999,28(5):361-364. |
| [3] | 马春树, 刘宇欣, 郑杰 , 等. 应用mRNA差异显示技术克隆肿瘤转移相关基因LASS2/TMSG1[J]. 中国科学, 2002,32(3):270-275. |
| [4] |
Strausberg RL, Feingold EA, Grouse LH , et al. Generation and initial analysis of more than 15,000 full-length human and mouse cDNA sequences[J]. Proc Natl Acad Sci USA, 2002,99(26):16899-16903.
doi: 10.1073/pnas.242603899 |
| [5] |
Pan H, Qin WX, Huo KK , et al. Cloning, mapping, and charaterization of a human homologue of the yeast longevity assurance gene LAG1[J]. Genomics, 2001,77(1/2):58-64.
doi: 10.1006/geno.2001.6614 |
| [6] |
Pewzner-Jung Y, Ben Dor S, Futerman AH . When do Lasses (longevity assurance genes) become CerS (ceramide synthases)? Insights into the regulation of ceramide synjournal[J]. J Biol Chem, 2006,281(35):25001-25005.
doi: 10.1074/jbc.R600010200 |
| [7] | 裴裴, 由江峰, 宁钧宇 , 等. 人肿瘤转移抑制基因TMSG-1单克隆抗体的制备、鉴定及在肿瘤检测中的应用[J]. 中华病理学杂志, 2005,34(1):15-21. |
| [8] |
Yu W, Wang L, Pei F , et al. A novel tumor metastasis suppressor gene LASS2/TMSG1 interacts with vacuolar ATPase through its homeodomain[J]. J Cell Biochem, 2013,114(3):570-583.
doi: 10.1002/jcb.v114.3 |
| [9] |
Ohta T, Numata M, Yagishita H , et al. Expression of 16 kDa proteolipid of vacuolar-type H(+)-ATPase in human pancreatic cancer[J]. Br J Cancer, 1996,73(12):1511-1517.
doi: 10.1038/bjc.1996.285 |
| [10] |
Sennoune SR, Bakunts K, Martinez GM , et al. Vacuolar H+-ATPase in human breast cancer cells with distinct metastatic potential: distribution and functional activity[J]. Am J Physiol Cell Physiol, 2004,286(6):1443-1452.
doi: 10.1152/ajpcell.00407.2003 |
| [11] |
Lee I, Skinner MA, Guo HB , et al. Expression of the vacuolar H+-ATPase 16-kDa subunit results in the Triton X-100-insoluble aggregation of beta1 integrin and reduction of its cell surface expression[J]. J Biol Chem, 2004,279(51):53007-53014.
doi: 10.1074/jbc.M405717200 |
| [12] |
Vitavska O, Wieczorek H, Merzendorfer H . A novel role for subunit C in mediating binding of the H+-V-ATPase to the actin cytoskeleton[J]. J Biol Chem, 2003,278(20):18499-18505.
doi: 10.1074/jbc.M212844200 |
| [13] |
Holliday LS, Lu M, Lee BS , et al. The amino-terminal domain of the B subunit of vacuolar H+-ATPase contains a filamentous actin binding site[J]. J Biol Chem, 2000,275(41):32331-32337.
doi: 10.1074/jbc.M004795200 |
| [14] |
Goldstein DJ, Andresson T, Sparkowski JJ , et al. The BPV-1 E5 protein, the 16 kDa membrane pore-forming protein and the PDGF receptor exist in a complex that is dependent on hydrophobic transmembrane interactions[J]. EMBO J, 1992,11(13):4851-4859.
doi: 10.1002/embj.1992.11.issue-13 |
| [15] |
Skinner MA , Wildeman AG. beta(1) integrin binds the 16-kDa subunit of vacuolar H(+)-ATPase at a site important for human papillomavirus E5 and platelet-derived growth factor signaling[J]. J Biol Chem, 1999,274(33):23119-23127.
doi: 10.1074/jbc.274.33.23119 |
| [16] |
Liotta LA, Kohn EC . The microenvironment of the tumour-host interface[J]. Nature, 2001,411(6835):375-379.
doi: 10.1038/35077241 |
| [17] | Zou P, Yang Y, Pei F , et al. Silencing of vacuolar ATPase c subunit ATP6V0C inhibits invasion of prostate cancer cell through LASS2/TMSG1 independent manner[J]. Oncol Rep, 2018,39(1):298-306. |
| [18] |
Kim SS, Chae HS, Bach JH , et al. P53 mediates ceramide-induced apoptosis in SKN-SH cells[J]. Oncogene, 2002,21(13):2020-2028.
doi: 10.1038/sj.onc.1205037 |
| [19] |
Kim HJ, Ghil KC, Kim MS , et al. Potentiation of ceramide-induced apoptosis by p27kip1 overexpression[J]. Arch Pharm Res, 2005,28(1):87-92.
doi: 10.1007/BF02975141 |
| [20] |
Yang H, Sadda MR, Li M , et al. S-adenosylmethionine and its metabolite induce apoptosis in HepG2 cells: Role of protein phosphatase 1 and Bcl-x(S)[J]. Hepatology, 2004,40(1):221-231.
doi: 10.1002/hep.v40:1 |
| [21] |
Lee JY, Bielawska AE, Obeid LM . Regulation of cyclin-depen-dent kinase 2 activity by ceramide[J]. Exp Cell Res, 2000,261(2):303-311.
doi: 10.1006/excr.2000.5028 |
| [22] |
Zhu XF, Liu ZC, Xie BF , et al. Ceramide induces cell cycle arrest and upregulates p27kip in nasopharyngeal carcinoma cells[J]. Cancer Lett, 2003,193(2):149-154.
doi: 10.1016/S0304-3835(03)00050-8 |
| [1] | Ye YAN,Xiaolong LI,Haizhui XIA,Xuehua ZHU,Yuting ZHANG,Fan ZHANG,Ke LIU,Cheng LIU,Lulin MA. Analysis of risk factors for long-term overactive bladder after radical prostatectomy [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 589-593. |
| [2] | Sheng-jie LIU,Hui-min HOU,Zheng-tong LV,Xin DING,Lu WANG,Lei ZHANG,Ming LIU. Bipolar androgen therapy followed by immune checkpoint inhibitors in metastatic castration resistant prostate cancer: A report of 4 cases [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 766-769. |
| [3] | BAI Gao-chen,SONG Yi,JIN Jie,YU Wei,HE Zhi-song. Clinical efficacy of docetaxel combined with carboplatin in patients with metastatic castration-resistant prostate cancer [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 686-691. |
| [4] | Kui-xia SUN,Cun-ling YAN,Zhi-yan LI,Ping LIU,Wei ZHANG,Qun HE. Clinical value of serum isoform [-2] proprostate-specific antigen and its derivatives in predicting aggressive prostate cancer [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 234-239. |
| [5] | Wen-qing LI,Si-mei REN,Xing-bo LONG,Yu-qing TIAN. Palmitoylome profiling indicates that androgens promote the palmitoylation of metabolism-related proteins in prostate cancer-derived LNCaP cells [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 227-233. |
| [6] | TANG Xu, ZHAO Wei-hong, SONG Qin-qin, YIN Hua-qi, DU Yi-qing, SHENG Zheng-zuo, WANG Qiang, ZHANG Xiao-wei, LI Qing, LIU Shi-jun, XU Tao. Influence of SOX10 on the proliferation and invasion of prostate cancer cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 602-606. |
| [7] | ZOU Peng-cheng, YANG Yi-feng, XU Xiao-yan LIU Bei-ying, MEI Fang, YOU Jiang-feng, LIU Qi-chen, PEI Fei . Silencing of vacuolar ATPase c subunit ATP6V0C inhibits invasion of prostate cancer cells [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 937-947. |
| [8] | JI Guang-jie, HUANG Cong, SONG Gang, LI Xue-song, SONG Yi, ZHOU Li-qun. Predictive factor analysis of time to progression of castration-resistant prostate cancer after androgen deprivation therapy [J]. Journal of Peking University(Health Sciences), 2017, 49(4): 657-662. |
|
||