Journal of Peking University(Health Sciences) ›› 2019, Vol. 51 ›› Issue (2): 245-251. doi: 10.19723/j.issn.1671-167X.2019.02.008
Previous Articles Next Articles
Pulsed electromagnetic fields stimulating osteogenic differentiation and maturation involves primary cilia-PI3K/AKT pathway
Qian REN1,Jian ZHOU1,Ming-gang WANG2,Ke-ming CHEN1,∆( )
)
- 1. Institute of Orthopaedics, Lanzhou General Hospital of PLA, Lanzhou 730050, China
2. College of Life Science and Engineering,Lanzhou University of Technology, Lanzhou 730050, China
CLC Number:
- R336
| [1] |
石文贵, 马小妮, 陈克明 . 初级纤毛在细胞信号转导中的作用与机制[J]. 浙江大学学报(医学版), 2014,43(3):359-365.
doi: 10.3785/j.issn.10089292.2014.04.006 |
| [2] |
Shi WG, Xie YF, He JP , et al. Microgravity induces inhibition of osteoblastic differentiation and mineralization through abrogating primary cilia[J]. Sci Rep, 2017,7(1):1866-1877.
doi: 10.1038/s41598-017-02049-9 |
| [3] |
Wanka H, Lutze P, Staar D , et al.( Pro)renin receptor (ATP6AP2) depletion arrests As4.1 cells in the G0/G1 phase thereby increasing formation of primary cilia[J]. J Cell Mol Med, 2017,21(7):13-19.
doi: 10.1111/jcmm.2017.21.issue-1 |
| [4] |
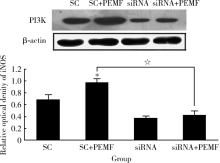
Zhai YK, Guo XY, Ge BF , et al. Icariin stimulates the osteogenic differentiation of rat bone marrow stromal cells via activating the PI3K-AKT-eNOS-NO-cGMP-PKG[J]. Bone, 2014,66(9):189-198.
doi: 10.1016/j.bone.2014.06.016 |
| [5] | Ehnert S, Sreekumar V, Asperawerz RH , et al. TGF-β1 impairs mechanosensation of human osteoblasts via HDAC6-mediated shortening and distortion of primary cilia[J]. J Mol Med, 2017,95(6):1-11. |
| [6] |
Kirschen GW, Liu H, Lang T , et al. The radial organization of neuronal primary cilia is acutely disrupted by seizure and ischemic brain injury[J]. Front Biol, 2017,12(2):1-15.
doi: 10.1007/s11515-016-1438-7 |
| [7] |
Akama KT , McEwen BS. Estrogen stimulates postsynaptic density-95 rapid protein synjournal via the Akt /protein kinase B pathway[J]. J Neurosci, 2003,23(6):2333-2339.
doi: 10.1523/JNEUROSCI.23-06-02333.2003 |
| [8] |
Markus A, Zhong J, Snider WD . Raf and akt mediate distinct aspects of sensory axon growth[J]. Neuron, 2002,35(1):65-76.
doi: 10.1016/S0896-6273(02)00752-3 |
| [9] | 孟金兰, 陈雅嘉, 陈红 , 等. PI3K/Akt 信号通路在硫化氢保护PC12细胞对抗化学性缺氧损伤的作用[J]. 中国药理学通报, 2013,29(2):257-261. |
| [10] |
Chen Y, Hu Y, Yang L , et al. Runx2 alleviates high glucose-suppressed osteogenic differentiation VIA PI3K/AKT/GSK3β/β-catenin pathway[J]. Cell Biol Int, 2017,41(8):822-832.
doi: 10.1002/cbin.v41.8 |
| [11] | Ma Y, Fu S, Lu L , et al. Role of androgen receptor on cyclic mechanical stretch-regulated proliferation of C2C12 myoblasts and its upstream signals: IGF-1-mediated PI3K/Akt and MAPKs pathways[J]. Mol Cell Endocrinol, 2017,45(8):83-93. |
| [12] |
Wang J, Ma XY, Feng YF , et al. Magnesium lons promote the biological behaviour of rat calvarial osteoblasts by activating the PI3K/Akt signalling pathway[J]. Biol Trace Elem Res, 2017,179(2):1-10.
doi: 10.1007/s12011-017-0938-x |
| [13] | Song F, Wang Y, Jiang D , et al. Cyclic compressive stress regulates apoptosis in rat osteoblasts: involvement of PI3K/Akt and JNK MAPK signaling pathways[J]. PLoS One, 2016,11(11):165-176. |
| [14] |
Zhou J, Ming LG, Ge BF , et al. Effects of 50 Hz sinusoidal electromagnetic fields of different intensities on proliferation differentiation and mineralization potentials of rat osteoblasts[J]. Bone, 2011,49(4):753-761
doi: 10.1016/j.bone.2011.06.026 |
| [15] | 高玉海, 成魁, 葛宝丰 , 等. 50Hz不同强度正弦交变电磁场对大鼠峰值骨量的影响[J]. 生物医学工程学杂志, 2015,13(1):116-119 |
| [16] | 任茜, 王鸣刚, 陈克明 , 等. 脉冲电磁场促进大鼠成骨细胞成熟与矿化依赖于NO/cGMP信号途径[J]. 中国生物化学与分子生物学报, 2016,32(11):1242-1248. |
| [17] | 方清清, 李志忠, 陈克明 , 等. 50Hz 1.8mT正弦交变电磁场通过调节成骨细胞PGE2分泌影响Opg/Rankl的基因表达[J]. 中国生物化学与分子生物学报, 2015,18(9):983-988. |
| [18] | Liu JM, Ning G, Chen JL . Osteoporotic fractures in Asia: risk factors and strategies for prevention[J]. J Bone Miner Metab, 2007,25(1):1-5. |
| [19] |
张辉, 李亮, 王茜 , 等. 骨形成蛋白-7对多孔钽/软骨细胞复合物分泌功能以及 Col-Ⅱ、AGG 和 Sox9基因表达的影响[J]. 北京大学学报(医学版), 2015,47(2):219-225.
doi: 10.3969/j.issn.1671-167X.2015.02.006 |
| [20] |
Paula FJ, Rosen CJ . Back to the future: revisiting parathyroid hormone and calcitonin control of bone remodeling[J]. Horm Metab Res, 2010,42(5):299-306.
doi: 10.1055/s-0030-1248255 |
| [21] |
Bonnelye E, Chabadel A, Saltel F , et al. Dual effect of strontium ranelate: stimulation of osteoblast differentiation and inhibition of osteoclast formation and resorption in vitro[J]. Bone, 2008,42(1):129-138.
doi: 10.1016/j.bone.2007.08.043 |
| [22] | 何伟, 李自力, 崔元璐 , 等. 淫羊藿苷对大鼠成骨细胞核结合因子α1、骨形成蛋白-2、骨形成蛋白-4 mRNA表达的影响[J]. 北京大学学报(医学版), 2009,41(6):669-673. |
| [23] | Bassett CA . The development and application of pulsed electromagnetic fields (PEMFs) for ununited fractures and arthrodeses[J]. Orthop Clin North Am, 1984,15(1):61-87. |
| [24] |
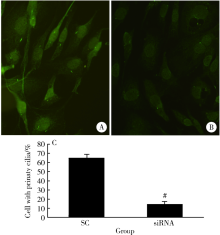
Spasic M, Jacobs CR . Lengthening primary cilia enhances cellular mechanosensitivity[J]. Eur Cells Master, 2017,33(2):158-169.
doi: 10.22203/eCM |
| [25] |
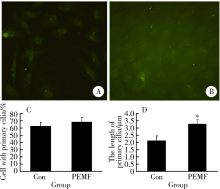
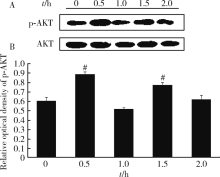
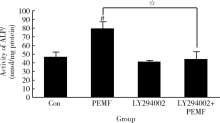
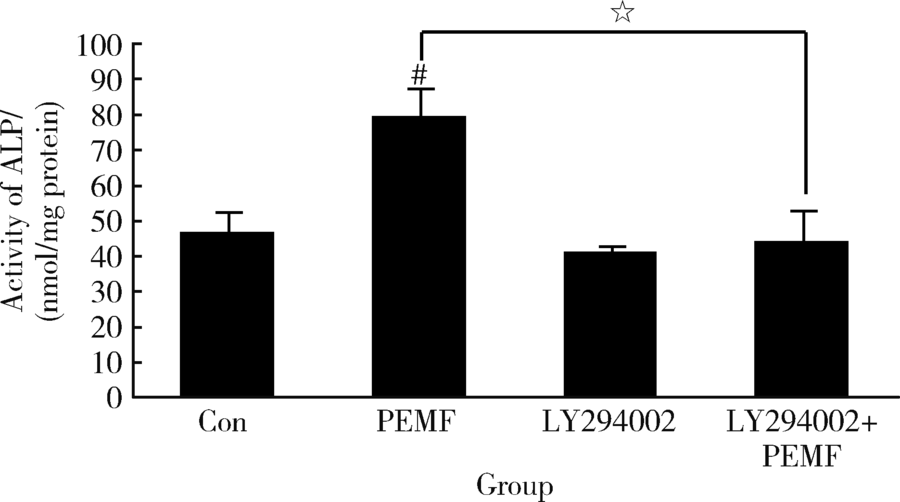
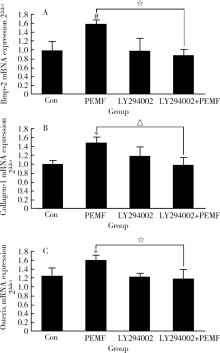
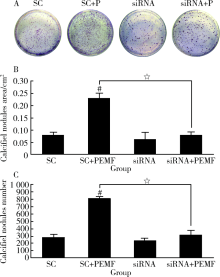
Yan JL, Zhou J, Ma HP , et al. Pulsed electromagnetic fields promote osteoblast mineralization and maturation needing the existence of primary cilia[J]. Mol Cell Endocrinol, 2015,404(3):132-140
doi: 10.1016/j.mce.2015.01.031 |
| [26] |
Xie YF, Shi WG, Zhou J , et al. Pulsed electromagnetic fields stimulate osteogenic differentiation and maturation of osteoblasts by upregulating the expression of BMPRⅡ localized at the base of primary cilium[J]. Bone, 2016,93(2):22-32.
doi: 10.1016/j.bone.2016.09.008 |
| [1] | WANG Zheng,DING Qian,GAO Yuan,MA Quan-quan,ZHANG Lei,GE Xi-yuan,SUN Yu-chun,XIE Qiu-fei. Effect of porous zirconia ceramics on proliferation and differentiation of osteoblasts [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 31-39. |
| [2] | LI Zheng,WANG Xiao,HONG Tian-pei,WANG Hao-jie,GAO Zhan-yi,WAN Meng. Mechanism of advanced glycation end products inhibiting the proliferation of peripheral blood mononuclear cells and osteoblasts in rats [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 355-363. |
| [3] | LI Man, LI Yuan, SUN Lin, SONG Jun-lai, LV Cong. High mobility group box 1 promotes apoptosis of astrocytes after oxygen glucose deprivation/reoxygenation by regulating the expression of Bcl-2 and Bax [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 785-791. |
| [4] | WANG Hao, CHEN Liang, YE Xiao-yun. Triptolide induces oxidative stress and apoptosis and activates PIK3/Akt signaling pathway in TM4 sertoli cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 607-612. |
| [5] | GAN Hong-quan, WANG Qian, ZHANG Hui, LIU Xin, DENG Hua-min, SONG Hui-ping, WANG ZHi-qiang, LI Qi-jia. Effects of RGD peptides-grafted porous tantalum on morphological change of MG63 osteoblasts-tantalum conjunctive interface and expression of osteogenesis factors [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 176-182. |
| [6] | LING Long, ZHAO Yu-ming, GE Li-hong. Impact of different degree pulpitis on cell proliferation and osteoblastic differentiation of dental pulp stem cell in Beagle immature premolars [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 878-883. |
| [7] | GE Xiao-dong, LI Mei-ling, WEN Xi-lin, LI Yi, DENG Xiao-lin, WU Xiao-feng, WEN Ming, LI Shao-lin. Optimal concentration of superparamagnetic iron oxide-short hairpin RNA dual functional molecular probe transfected into ovarian cancer cells in vitro [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 754-760. |
|
||