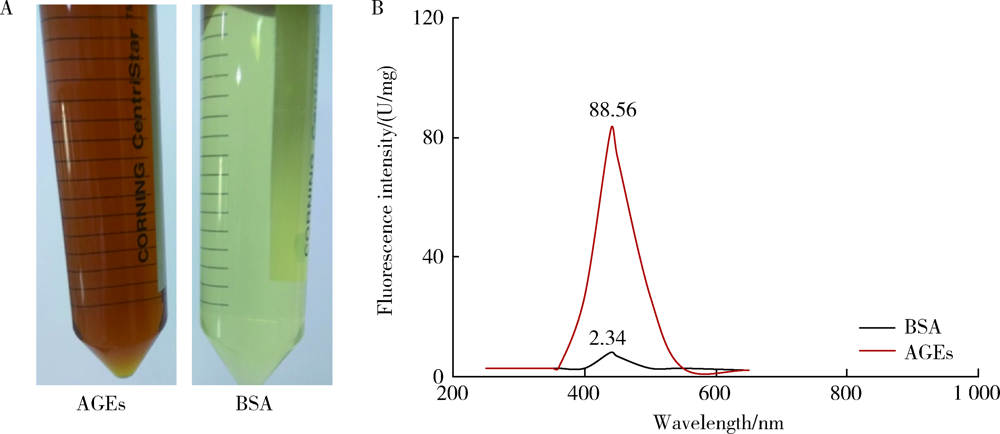
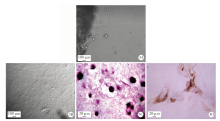
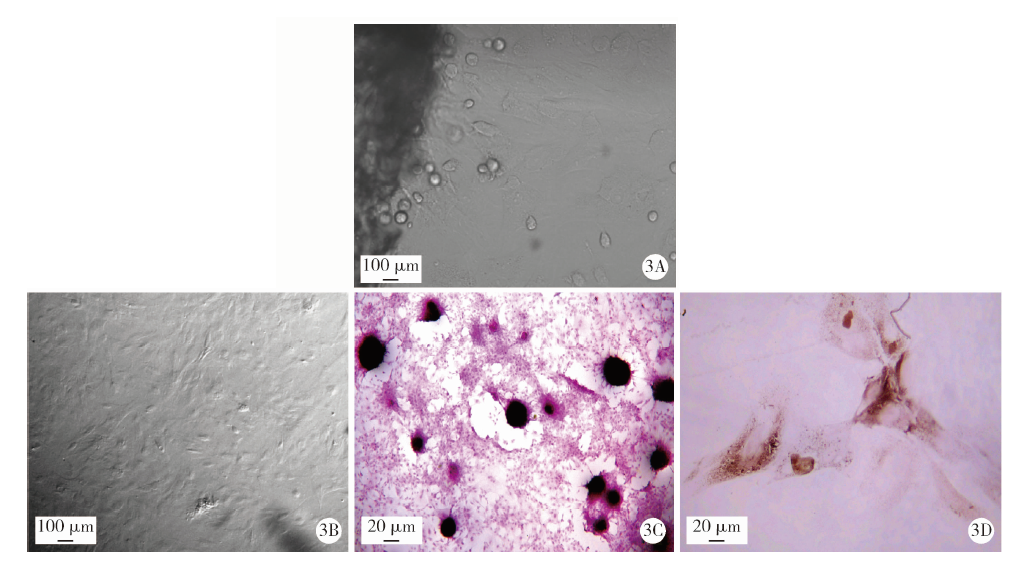
Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (2): 355-363. doi: 10.19723/j.issn.1671-167X.2021.02.021
Previous Articles Next Articles
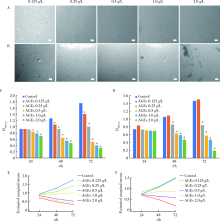
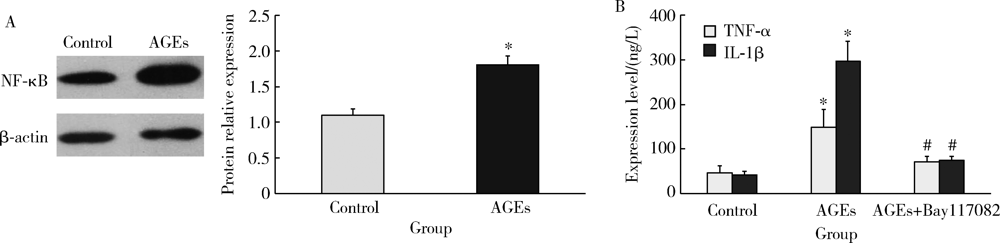
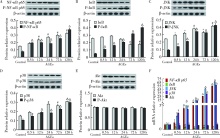
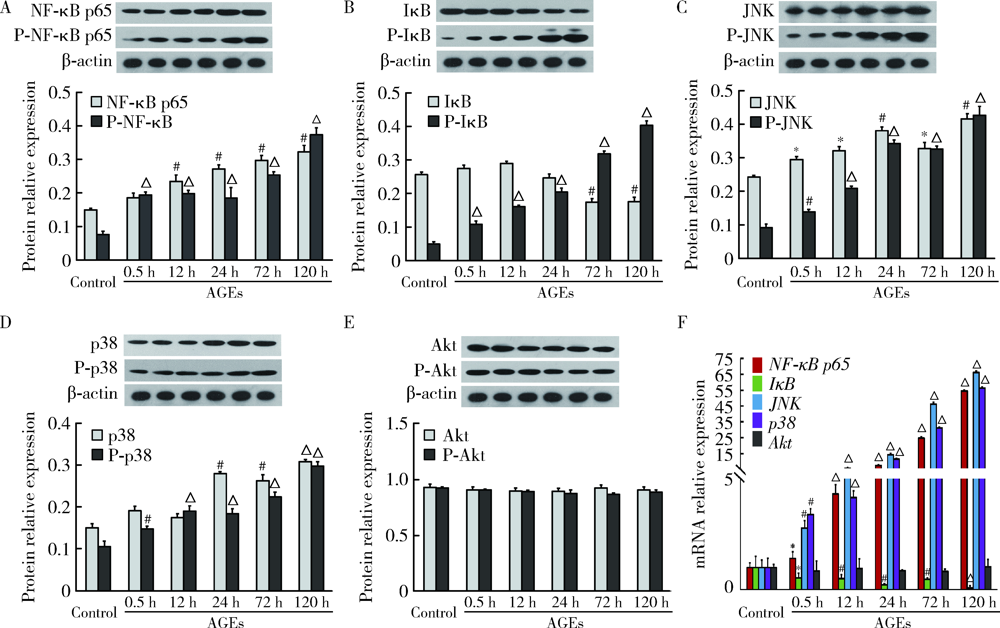
Mechanism of advanced glycation end products inhibiting the proliferation of peripheral blood mononuclear cells and osteoblasts in rats
LI Zheng1,WANG Xiao1,Δ( ),HONG Tian-pei2,WANG Hao-jie1,GAO Zhan-yi1,WAN Meng1
),HONG Tian-pei2,WANG Hao-jie1,GAO Zhan-yi1,WAN Meng1
- 1. Department of Stomatology, Peking University Third Hospital, Beijing 100191, China
2. Department of Endocrinology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R781.4
| [1] |
Petersen PE, Ogawa H. The global burden of periodontal disease: Towards integration with chronic disease prevention and control[J]. Periodontol 2000, 2012,60(1):15-39.
doi: 10.1111/j.1600-0757.2011.00425.x pmid: 22909104 |
| [2] | 李峥, 沙月琴, 张博学, 等. 参加社区慢性病管理的糖尿病患者牙周健康状况调查及相关因素分析[J]. 中华口腔医学杂志, 2007: 42(2):100-101. |
| [3] | Papapanou PN. Periodontal disease: Epidemiology[J]. Annals of Periodontol, 1996,1(1):11-36. |
| [4] |
Khader YS, Dauod AS, El-Qaderi SS, et al. Periodontal status of diabetics compared with nondiabetics: A meta-analysis[J]. J Diabetes Complications, 2006,20(1):59-68.
pmid: 16389170 |
| [5] |
Taylor JJ, Preshaw PM, Lalla E. A review of the evidence for pathogenic mechanisms that may link periodontitis and diabetes[J]. J Periodontol, 2013,84(4 Suppl.):S113-S134.
pmid: 23631573 |
| [6] |
Taiyeb-Ali TB, Cheta Raman RP, Vaithilingam RD. Relationship between periodontal disease and diabetes mellitus: An Asian perspective[J]. Periodontol 2000, 2011,56(1):258-268.
pmid: 21501247 |
| [7] | Ulrich P, Cerami A. Protein glycation, diabetes, and aging[J]. Recent Prog Horm Res, 2001,56(1):1-21. |
| [8] | Ramasamy R, Vannucci SJ, Yan SS, et al. Advanced glycation end products and RAGE: A common thread in aging, diabetes, neurodegeneration, and inflammation[J]. Glycobiology, 2005,15(7):16R-28R. |
| [9] |
Neeper M, Schmidt AM, Brett J, et al. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins[J]. J Biol Chem, 1992,267(21):14998-15004.
pmid: 1378843 |
| [10] | Southerlan JH, Taylor GW, Moss K, et al. Commonality in chro-nic inflammatory diseases: Periodontitis, diabetes, and coronary artery disease[J]. Periodontol 2000, 2006,40(1):130-143. |
| [11] |
Lin L. RAGE on the Toll Road?[J]. Cell Mol Immunol, 2006,3(5):351-358.
pmid: 17092432 |
| [12] |
Schmidt A, Yan S, Brett J, et al. Regulation of human mononuclear phagocyte migration by cell surface-binding proteins for advanced glycation end products[J]. J Clin Invest, 1993,91(5):2155-2168.
doi: 10.1172/JCI116442 pmid: 8387541 |
| [13] |
Anfelika B, Stephan S, Markus S, et al. Diabetes-associated sustained activation of the transcription factor nuclear factor-kappa B[J]. Diabetes, 2001,50(12):2792-2808.
pmid: 11723063 |
| [14] |
Chen Q, Dong L, Wang L, et al. Advanced glycation end pro-ducts impair function of late endothelial progenitor cells through effects on protein kinase Akt and cyclooxygenase-2[J]. Biochem Biophys Res Commun, 2009,381(2):192-197.
pmid: 19232321 |
| [1] | Yang-yang GU,Xiao-hui TAN,Wen-peng SONG,Dong FANG,Wei-dong SONG,Yi-ming YUAN,Ning-han FENG,Rui-li GUAN. Effects of 4′-O-methylochnaflavone on endothelial dysfunction induced by palmitic acid in rat cavernous endothelial cells [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 599-604. |
| [2] | WANG Zheng,DING Qian,GAO Yuan,MA Quan-quan,ZHANG Lei,GE Xi-yuan,SUN Yu-chun,XIE Qiu-fei. Effect of porous zirconia ceramics on proliferation and differentiation of osteoblasts [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 31-39. |
| [3] | XIAO Ruo-tao,LIU Cheng,XU Chu-xiao,HE Wei,MA Lu-lin. Prognostic value of preoperative platelet parameters in locally advanced renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 647-652. |
| [4] | Jia-yu WANG,Mei-ping ZHAO. Fluorescence assay for the detection of apurinic/apyrimidinic endonuclease 1 (APE1) activity in human blood samples [J]. Journal of Peking University(Health Sciences), 2019, 51(3): 487-492. |
| [5] | GAN Hong-quan, WANG Qian, ZHANG Hui, LIU Xin, DENG Hua-min, SONG Hui-ping, WANG ZHi-qiang, LI Qi-jia. Effects of RGD peptides-grafted porous tantalum on morphological change of MG63 osteoblasts-tantalum conjunctive interface and expression of osteogenesis factors [J]. Journal of Peking University(Health Sciences), 2018, 50(1): 176-182. |
| [6] | GUO Qian, CHEN Xu-yong, SU Yin. Interleukin-2 signaling pathway regulating molecules in systemic lupus erythematosus [J]. Journal of Peking University(Health Sciences), 2016, 48(6): 1100-1104. |
| [7] | LING Long, ZHAO Yu-ming, GE Li-hong. Impact of different degree pulpitis on cell proliferation and osteoblastic differentiation of dental pulp stem cell in Beagle immature premolars [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 878-883. |
| [8] | XIAO Yang, DU Yao-yao, GAO Cheng, KONG Wei. Dynamic alteration of microRNA in high phosphorus induced calcification of vascular smooth muscle cell [J]. Journal of Peking University(Health Sciences), 2016, 48(5): 756-765. |
| [9] | CAO Pei, JIANG Xue-jun, XI Zhi-jun. Sunitinib induces autophagy via suppressing Akt/mTOR pathway in renal cell carcinoma [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 584-589. |
| [10] | WANG Zhu-Qing, WANG Ping, WU-CHOU Yah-huei, YE Xiao-Qian, HUANG Shang-Zhi, SHI Bing, WANG Ke, YUAN Yuan, LIU Dong-Jing, WU Tao, WANG Hong, Terri H. Beaty. Association study between candidate genes on transforming growth factor-β signaling pathway and the risk of non-syndromic cleft lip with or without cleft palate in Chinese populations [J]. Journal of Peking University(Health Sciences), 2015, 47(3): 384-389. |
| [11] | Li-Jing-Jin, CHEN Hong, REN Jing-Yi. Targeted effect of microRNA on nerve growth factor pathway and its functional network in patients with unstable angina [J]. Journal of Peking University(Health Sciences), 2014, 46(6): 868-874. |
| [12] | CHANG Si-jia, ZOU Xiao-ying, ZHUANG Heng, YUE Lin, GAO Xue-jun. Notch activation delayed ageing of human dental pulp cells [J]. Journal of Peking University(Health Sciences), 2014, 46(1): 5-11. |
|
||