Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (4): 647-652. doi: 10.19723/j.issn.1671-167X.2021.04.004
Previous Articles Next Articles
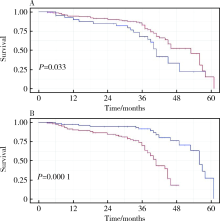
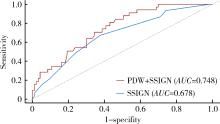
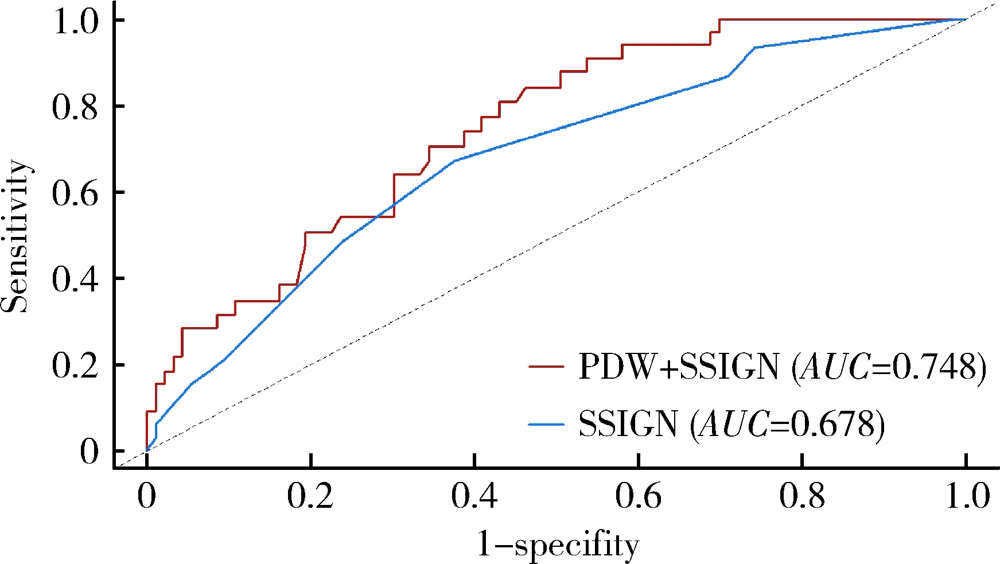
Prognostic value of preoperative platelet parameters in locally advanced renal cell carcinoma
XIAO Ruo-tao,LIU Cheng,XU Chu-xiao,HE Wei,MA Lu-lin( )
)
- Department of Urology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R737.11
| [1] |
Gay LJ, Felding-Habermann B. Contribution of platelets to tumour metastasis [J]. Nat Rev Cancer, 2011, 11(2):123-134.
doi: 10.1038/nrc3004 |
| [2] |
Labelle M, Begum S, Hynes RO. Direct signaling between platelets and cancer cells induces an epithelial-mesenchymal-like transition and promotes metastasis [J]. Cancer Cell, 2011, 20(5):576-590.
doi: 10.1016/j.ccr.2011.09.009 pmid: 22094253 |
| [3] |
Nieswandt B, Hafner M, Echtenacher B, et al. Lysis of tumor cells by natural killer cells in mice is impeded by platelets [J]. Cancer Res, 1999, 59(6):1295-1300.
pmid: 10096562 |
| [4] |
Zhu X, Cao Y, Lu P, et al. Evaluation of platelet indices as diagnostic biomarkers for colorectal cancer [J]. Sci Rep, 2018, 8(1):11814.
doi: 10.1038/s41598-018-29293-x |
| [5] |
Liu C, Zhang H, Qi Q, et al. The preoperative platelet distribution width: A predictive factor of the prognosis in patients with non-small cell lung cancer [J]. Thorac Cancer, 2020, 11(4):918-927.
doi: 10.1111/tca.v11.4 |
| [6] |
Liu S, Fang J, Jiao D, et al. Elevated platelet count predicts poor prognosis in breast cancer patients with supraclavicular lymph node metastasis [J]. Cancer Manag Res, 2020, 12(6):6069-6075.
doi: 10.2147/CMAR.S257727 |
| [7] |
Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the international metastatic renal-cell carcinoma database consortium prognostic model: a population-based study [J]. Lancet Oncol, 2013, 14(2):141-148.
doi: 10.1016/S1470-2045(12)70559-4 |
| [8] |
Choi JY, Ko YH, Song PH. Clinical significance of preoperative thrombocytosis in patients who underwent radical nephrectomy for nonmetastatic renal cell carcinoma [J]. Investig Clin Urol, 2016, 57(5):324-329.
doi: 10.4111/icu.2016.57.5.324 |
| [9] |
Seles M, Posch F, Pichler GP, et al. Blood platelet volume represents a novel prognostic factor in patients with nonmetastatic renal cell carcinoma and improves the predictive ability of established prognostic scores [J]. J Urol, 2017, 198(6):1247-1252.
doi: 10.1016/j.juro.2017.07.036 |
| [10] |
Karakiewicz PI, Trinh QD, Lam JS, et al. Platelet count and preoperative haemoglobin do not significantly increase the performance of established predictors of renal cell carcinoma-specific mortality [J]. Eur Urol, 2007, 52(5):1428-1436.
pmid: 17420085 |
| [11] |
Frank I, Blute ML, Cheville JC, et al. An outcome prediction model for patients with clear cell renal cell carcinoma treated with radical nephrectomy based on tumor stage, size, grade and necrosis: the SSIGN score [J]. J Urol, 2002, 168(6):2395-2400.
doi: 10.1016/S0022-5347(05)64153-5 |
| [12] |
Zisman A, Pantuck AJ, Dorey F, et al. Improved prognostication of renal cell carcinoma using an integrated staging system [J]. J Clin Oncol, 2001, 19(6):1649-1657.
pmid: 11250993 |
| [13] | Amin MB, Edge SB, Greene FL, et al. AJCC cancer staging manual[M]. 8th ed. Chicago: Springer, 2017: 739-747. |
| [14] |
Ljungberg B, Bensalah K, Canfield S, et al. EAU guidelines on renal cell carcinoma: 2014 update [J]. Eur Urol, 2015, 67(5):913-924.
doi: 10.1016/j.eururo.2015.01.005 pmid: 25616710 |
| [15] |
Moch H, Cubilla AL, Humphrey PA, et al. The 2016 WHO classification of tumours of the urinary system and male genital organs-part A: renal, penile, and testicular tumours [J]. Eur Urol, 2016, 70(1):93-105.
doi: 10.1016/j.eururo.2016.02.029 |
| [16] |
Siegel RL, Miller KD, Fuchs HE, et al. Cancer statistics, 2021 [J]. CA Cancer J Clin, 2021, 71(1):7-33.
doi: 10.3322/caac.v71.1 |
| [17] |
Haas NB, Manola J, Dutcher JP, et al. Adjuvant treatment for high-risk clear cell renal cancer: updated results of a high-risk subset of the ASSURE randomized trial [J]. JAMA Oncol, 2017, 3(9):1249-1252.
doi: 10.1001/jamaoncol.2017.0076 |
| [18] |
Motzer RJ, Haas NB, Donskov F, et al. Randomized phase Ⅲ trial of adjuvant pazopanib versus placebo after nephrectomy in patients with localized or locally advanced renal cell carcinoma [J]. J Clin Oncol, 2017, 35(35):3916-3923.
doi: 10.1200/JCO.2017.73.5324 |
| [19] |
Motzer RJ, Ravaud A, Patard JJ, et al. Adjuvant sunitinib for high-risk renal cell carcinoma after nephrectomy: subgroup analyses and updated overall survival results [J]. Eur Urol, 2018, 73(1):62-68.
doi: 10.1016/j.eururo.2017.09.008 |
| [20] |
Leibovich BC, Blute ML, Cheville JC, et al. Prediction of progression after radical nephrectomy for patients with clear cell renal cell carcinoma: a stratification tool for prospective clinical trials [J]. Cancer, 2003, 97(7):1663-1671.
pmid: 12655523 |
| [21] |
Karakiewicz PI, Briganti A, Chun FK, et al. Multi-institutional validation of a new renal cancer-specific survival nomogram [J]. J Clin Oncol, 2007, 25(11):1316-1322.
pmid: 17416852 |
| [22] |
Correa AF, Jegede O, Haas NB, et al. Predicting renal cancer recurrence: defining limitations of existing prognostic models with prospective trial-based validation [J]. J Clin Oncol, 2019, 37(23):2062-2071.
doi: 10.1200/JCO.19.00107 |
| [23] |
Xiao R, Xu C, He W, et al. Preoperative anaemia and thrombocytosis predict adverse prognosis in non-metastatic renal cell carcinoma with tumour thrombus [J]. BMC Urol, 2021, 21(1):31.
doi: 10.1186/s12894-021-00796-6 |
| [24] |
Yue CX, Liu YX, Yun ZY, et al. Decreased platelet distribution width predicts a worse prognosis in patients undergoing surgical resection for hepatocellular carcinoma [J]. Cancer Biomark, 2019, 26(3):361-366.
doi: 10.3233/CBM-190474 |
| [25] |
Chen H, Wu Q, Zhang Y, et al. Nomograms based on the novel platelet index score predict postoperative prognosis in endometrial cancer [J]. Gynecol Oncol, 2020, 158(3):689-697.
doi: S0090-8258(20)31125-2 pmid: 32507649 |
| [26] |
Kawakita Y, Motoyama S, Sato Y, et al. Prognostic significance of combined platelet distribution width and C-reactive protein score in esophageal cancer [J]. Anticancer Res, 2020, 40(10):5715-5725.
doi: 10.21873/anticanres.14586 pmid: 32988897 |
| [27] | 蒋慧云, 李小毛, 王佳, 等. 术前血小板分布宽度在子宫内膜癌诊断预测中的价值 [J]. 实用医学杂志, 2018, 34(7):1188-1190. |
| [28] |
Vagdatli E, Gounari E, Lazaridou E, et al. Platelet distribution width: a simple, practical and specific marker of activation of coagulation [J]. Hippokratia, 2010, 14(1):28-32.
pmid: 20411056 |
| [29] | 张翔, 庄瑞. 血小板分布宽度对鼻咽癌患者预后的影响 [J]. 国际肿瘤学杂志, 2018, 45(5):257-261. |
| [30] |
Huang Y, Cui MM, Huang YX, et al. Preoperative platelet distribution width predicts breast cancer survival [J]. Cancer Biomark, 2018, 23(2):205-211.
doi: 10.3233/CBM-181267 pmid: 30198864 |
| [31] | 张林楠, 刘玉峰, 苏淑芳, 等. 血小板分布宽度对神经母细胞瘤预后的预测价值 [J]. 中华实用儿科临床杂志, 2020, 35(6):440-444. |
| [1] | Junyong OU,Kunming NI,Lulin MA,Guoliang WANG,Ye YAN,Bin YANG,Gengwu LI,Haodong SONG,Min LU,Jianfei YE,Shudong ZHANG. Prognostic factors of patients with muscle invasive bladder cancer with intermediate-to-high risk prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 582-588. |
| [2] | Shuai LIU,Lei LIU,Zhuo LIU,Fan ZHANG,Lulin MA,Xiaojun TIAN,Xiaofei HOU,Guoliang WANG,Lei ZHAO,Shudong ZHANG. Clinical treatment and prognosis of adrenocortical carcinoma with venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 624-630. |
| [3] | Le YU,Shaohui DENG,Fan ZHANG,Ye YAN,Jianfei YE,Shudong ZHANG. Clinicopathological characteristics and prognosis of multilocular cystic renal neoplasm of low malignant potential [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 661-666. |
| [4] | Fan SHU,Yichang HAO,Zhanyi ZHANG,Shaohui DENG,Hongxian ZHANG,Lei LIU,Guoliang WANG,Xiaojun TIAN,Lei ZHAO,Lulin MA,Shudong ZHANG. Functional and oncologic outcomes of partial nephrectomy for cystic renal cell carcinoma: A single-center retrospective study [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 667-672. |
| [5] | Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG. Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 673-679. |
| [6] | Yangyi FANG,Qiang LI,Zhigao HUANG,Min LU,Kai HONG,Shudong ZHANG. Well-differentiated papillary mesothelial tumour of the tunica vaginalis: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 741-744. |
| [7] | Yuanyuan ZENG,Yun XIE,Daonan CHEN,Ruilan WANG. Related factors of euthyroid sick syndrome in patients with sepsis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 526-532. |
| [8] | Jian-bin LI,Meng-na LYU,Qiang CHI,Yi-lin PENG,Peng-cheng LIU,Rui WU. Early prediction of severe COVID-19 in patients with Sjögren’s syndrome [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1007-1012. |
| [9] | Yun-chong LIU,Zong-long WU,Li-yuan GE,Tan DU,Ya-qian WU,Yi-meng SONG,Cheng LIU,Lu-lin MA. Mechanism of nuclear protein 1 in the resistance to axitinib in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 781-792. |
| [10] | Huan-rui LIU,Xiang PENG,Sen-lin LI,Xin GOU. Risk modeling based on HER-2 related genes for bladder cancer survival prognosis assessment [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 793-801. |
| [11] | Zi-xuan XUE,Shi-ying TANG,Min QIU,Cheng LIU,Xiao-jun TIAN,Min LU,Jing-han DONG,Lu-lin MA,Shu-dong ZHANG. Clinicopathologic features and prognosis of young renal tumors with tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 802-811. |
| [12] | Dong LAN,Zhuo LIU,Yu-xuan LI,Guo-liang WANG,Xiao-jun TIAN,Lu-lin MA,Shu-dong ZHANG,Hong-xian ZHANG. Risk factors for massive hemorrhage after radical nephrectomy and removal of venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 825-832. |
| [13] | Han LU,Jian-yun ZHANG,Rong YANG,Le XU,Qing-xiang LI,Yu-xing GUO,Chuan-bin GUO. Clinical factors affecting the prognosis of lower gingival squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 702-707. |
| [14] | Yun-fei SHI,Hao-jie WANG,Wei-ping LIU,Lan MI,Meng-ping LONG,Yan-fei LIU,Yu-mei LAI,Li-xin ZHOU,Xin-ting DIAO,Xiang-hong LI. Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 521-529. |
| [15] | Yun-yi XU,Zheng-zheng SU,Lin-mao ZHENG,Meng-ni ZHANG,Jun-ya TAN,Ya-lan YANG,Meng-xin ZHANG,Miao XU,Ni CHEN,Xue-qin CHEN,Qiao ZHOU. Read-through circular RNA rt-circ-HS promotes hypoxia inducible factor 1α expression and renal carcinoma cell proliferation, migration and invasiveness [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 217-227. |
|
||