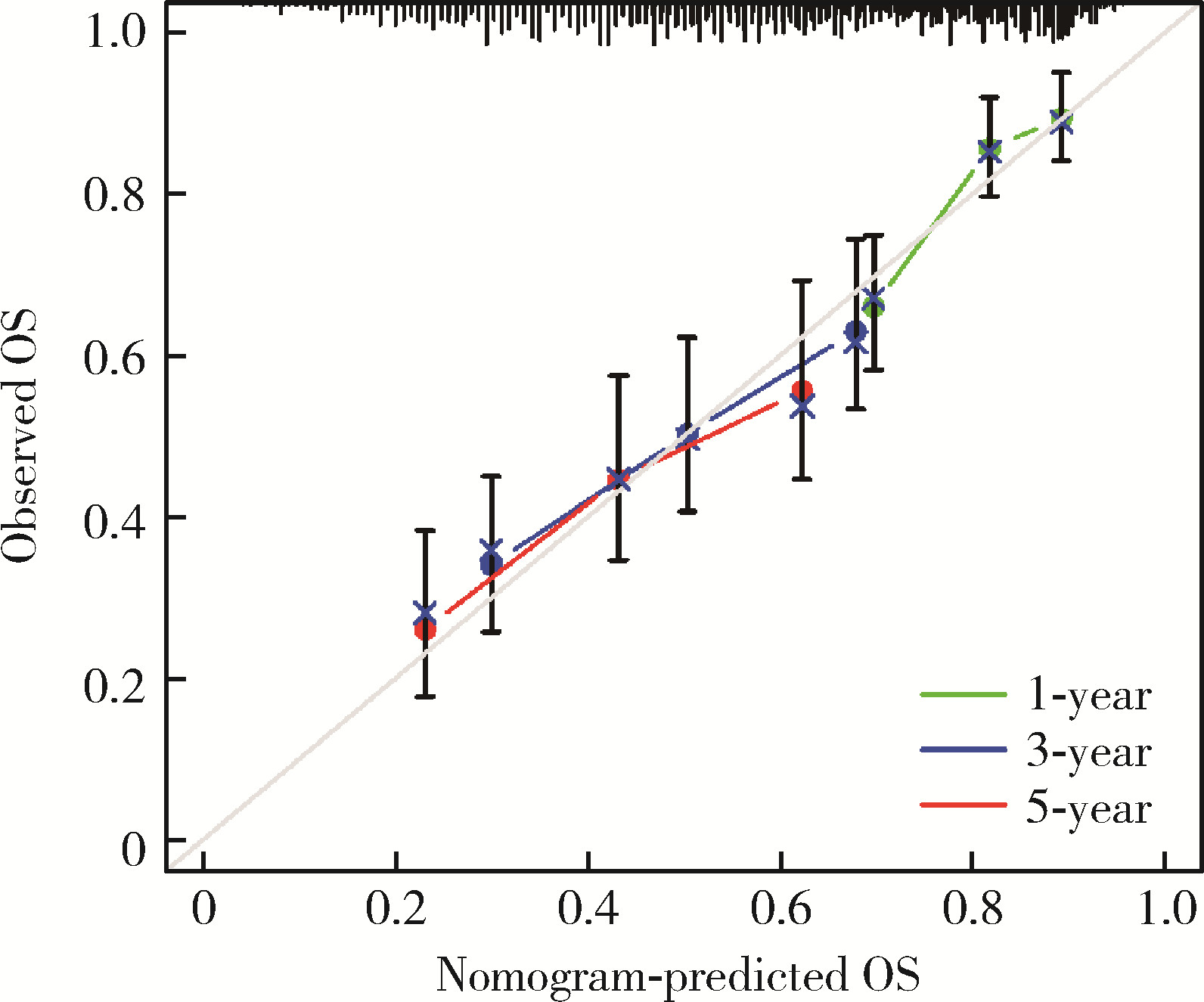
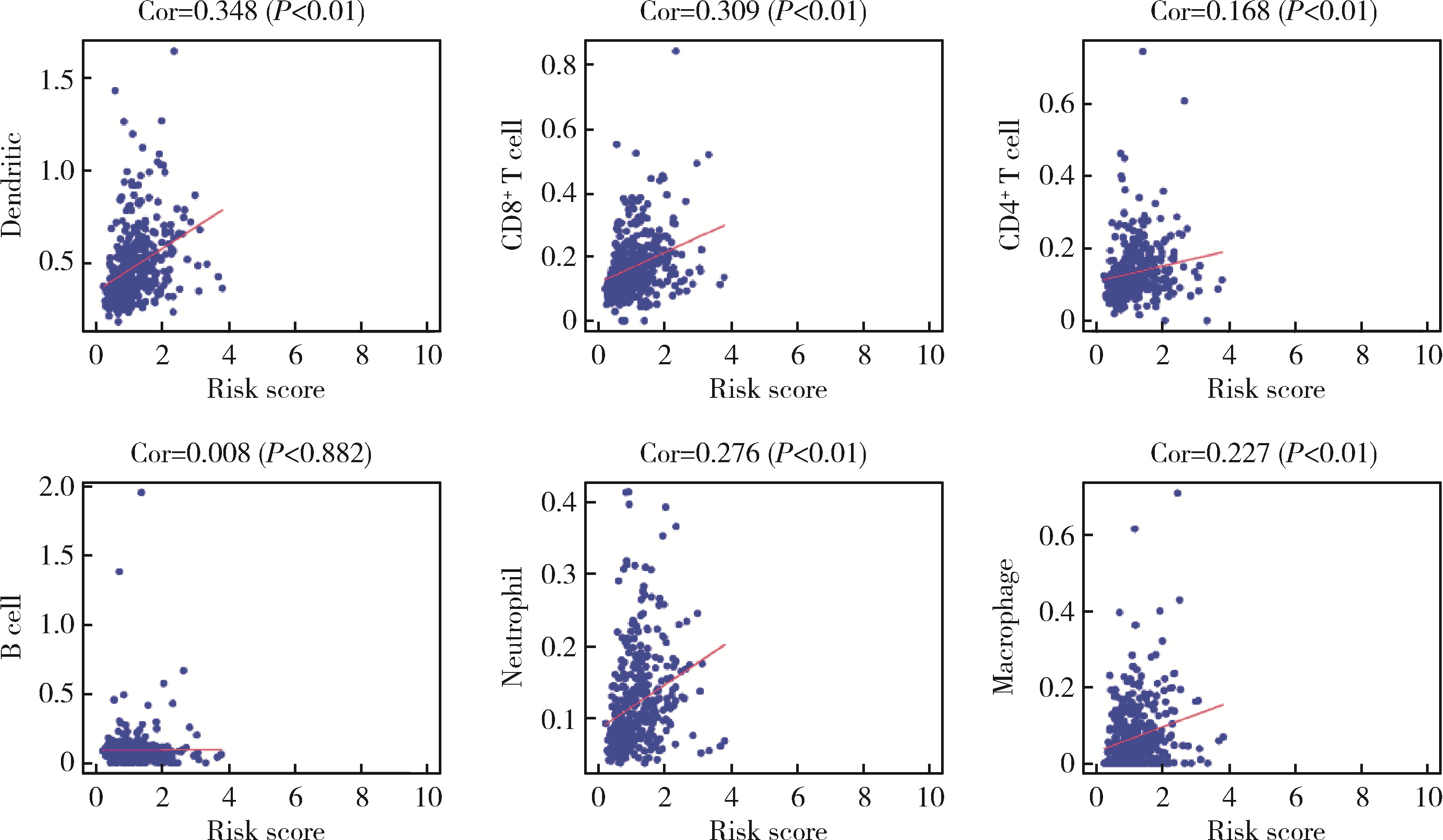
Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (5): 793-801. doi: 10.19723/j.issn.1671-167X.2023.05.004
Previous Articles Next Articles
Risk modeling based on HER-2 related genes for bladder cancer survival prognosis assessment
Huan-rui LIU1,Xiang PENG1,2,Sen-lin LI1,Xin GOU1,*( )
)
- 1. Department of Urology, The First Hospital of Chongqing Medical University, Chongqing 400016, China
2. Chongqing Key Laboratory of Molecular Tumor and Epigenetics, Chongqing 400016, China
CLC Number:
- R737.1
| 1 |
AneoniS , FerlayJ , SoerjomatarmI ,et al.Bladder cancer incidence and mortality: A global overview and recent trends[J].Eur Urol,2017,71(1):96-108.
doi: 10.1016/j.eururo.2016.06.010 |
| 2 |
NielsenME , SmithAB , MererAM ,et al.Trends in stage-specific incidence rates for urothelial carcinoma of the bladder in the Uni-ted States: 1988 to 2006[J].Cancer,2014,120(1):86-95.
doi: 10.1002/cncr.28397 |
| 3 |
CrispenPL , KusmartsevS .Mechanisms of immune evasion in bladder cancer[J].Cancer Immunol Immunother,2020,69(1):3-14.
doi: 10.1007/s00262-019-02443-4 |
| 4 |
Goossens-LaanCA , LeliveldAM , VerhoevenRH ,et al.Effects of age and comorbidity on treatment and survival of patients with muscle-invasive bladder cancer[J].Int J Cancer,2014,135(4):905-912.
doi: 10.1002/ijc.28716 |
| 5 | Redondo-GonzalezE , de CastroLN , Moreno-SierraJ ,et al.Bladder carcinoma data with clinical risk factors and molecular mar-kers: A cluster analysis[J].Biomed Res Int,2015,2015,168682. |
| 6 |
MoasserMM .The oncogene HER2 : Its signaling and transforming functions and its role in human cancer pathogenesis[J].Oncogene,2007,26(45):6469-6487.
doi: 10.1038/sj.onc.1210477 |
| 7 |
BegnamiMD , FukudaE , FregnaniJH ,et al.Prognostic implications of altered human epidermal growth factor receptors (HERs) in gastric carcinomas: HER2 and HER3 are predictors of poor outcome[J].J Clin Oncol,2011,29(22):3030-3036.
doi: 10.1200/JCO.2010.33.6313 |
| 8 |
BangYJ , van CutsemE , FeyereislovaA ,et al.Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial[J].Lancet,2010,376(9742):687-697.
doi: 10.1016/S0140-6736(10)61121-X |
| 9 |
WolffAC , HammondME , HicksDG ,et al.Recommendations for human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Patho-logists clinical practice guideline update[J].J Clin Oncol,2013,31(31):3997-4013.
doi: 10.1200/JCO.2013.50.9984 |
| 10 |
IyerG , Al-AhmadieH , SchultzN ,et al.Prevalence and co-occurrence of actionable genomic alterations in high-grade bladder cancer[J].J Clin Oncol,2013,31(25):3133-3140.
doi: 10.1200/JCO.2012.46.5740 |
| 11 |
BaiX , HeW , YinH ,et al.Prognostic significance of HER2 status evaluation using immunohistochemistry in patients with urothelial carcinoma of the bladder: A retrospective single-center experience[J].Exp Ther Med,2022,24(5):704.
doi: 10.3892/etm.2022.11640 |
| 12 |
LoiS , SirtaineN , PietteF ,et al.Prognostic and predictive value of tumor-infiltrating lymphocytes in a phase Ⅲ randomized adjuvant breast cancer trial in node-positive breast cancer comparing the addition of docetaxel to doxorubicin with doxorubicin-based chemotherapy: BIG 02-98[J].J Clin Oncol,2013,31(7):860-867.
doi: 10.1200/JCO.2011.41.0902 |
| 13 |
StantonSE , AdamsS , DisisML .Variation in the incidence and magnitude of tumor-infiltrating lymphocytes in breast cancer subtypes: A systematic review[J].JAMA Oncol,2016,2(10):1354-1360.
doi: 10.1001/jamaoncol.2016.1061 |
| 14 |
JiaY , KodumudiKN , RamamoorthiG ,et al.Th1 cytokine interferon gamma improves response in HER2 breast cancer by modulating the ubiquitin proteasomal pathway[J].Mol Ther,2021,29(4):1541-1556.
doi: 10.1016/j.ymthe.2020.12.037 |
| 15 |
WülfingC , MachielsJP , RichelDJ ,et al.A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma[J].Cancer,2009,115(13):2881-2890.
doi: 10.1002/cncr.24337 |
| 16 |
ShengX , YanX , WangL ,et al.Open-label, multicenter, phase Ⅱ study of RC48-ADC, a HER2-targeting antibody-drug conjugate, in patients with locally advanced or metastatic urothelial carcinoma[J].Clin Cancer Res,2021,27(1):43-51.
doi: 10.1158/1078-0432.CCR-20-2488 |
| 17 | ShengX , ZhouAP , YaoX ,et al.A phase Ⅱ study of RC48-ADC in HER2-positive patients with locally advanced or metastatic urothelial carcinoma[J].J Clin Oncol,2019,37(Suppl 15):4509. |
| 18 |
RushJS , PetersonJL , CeresaBP .Betacellulin (BTC) biases the EGFR to dimerize with ErbB3[J].Mol Pharmacol,2018,94(6):1382-1390.
doi: 10.1124/mol.118.113399 |
| 19 |
ShenT , YangT , YaoM ,et al.BTC as a novel biomarker contri-buting to EMT via the PI3K-AKT pathway in OSCC[J].Front Genet,2022,13,875617.
doi: 10.3389/fgene.2022.875617 |
| 20 |
DahlhoffM , WolfE , SchneiderMR .The ABC of BTC: Structural properties and biological roles of betacellulin[J].Semin Cell Dev Biol,2014,28,42-48.
doi: 10.1016/j.semcdb.2014.01.002 |
| 21 |
OlsenDA , KjaerIM , BrandslundI .Development of a three-plex single molecule immunoassay enabling measurement of the EGFR ligands amphiregulin, betacellulin and transforming growth factor alpha simultaneously in human serum samples[J].J Immunol Methods,2018,459,63-69.
doi: 10.1016/j.jim.2018.05.002 |
| 22 | LeeYS , SongGJ , JunHS .Betacellulin-induced alpha-cell proli-feration is mediated by ErbB3 and ErbB4, and may contribute to beta-cell regeneration[J].Front Cell Dev Biol,2020,8,605110. |
| 23 | OlsenDA , BechmannT , ϕstergaardB ,et al.Increased concentrations of growth factors and activation of the EGFR system in breast cancer[J].Clin Chem Lab Med,2012,50(10):1809-1818. |
| 24 |
PearlLH .Hsp90 and Cdc37: A chaperone cancer conspiracy[J].Curr Opin Genet Dev,2005,15(1):55-61.
doi: 10.1016/j.gde.2004.12.011 |
| 25 |
SerwetnykMA , BlaggBSJ .The disruption of protein-protein interactions with co-chaperones and client substrates as a strategy towards Hsp90 inhibition[J].Acta Pharm Sin B,2021,11(6):1446-1468.
doi: 10.1016/j.apsb.2020.11.015 |
| 26 |
GrayPJ , Jr. , PrinceT , ChengJ ,et al.Targeting the oncogene and kinome chaperone CDC37[J].Nat Rev Cancer,2008,8(7):491-495.
doi: 10.1038/nrc2420 |
| 27 |
GhatakS , MisraS , TooleBP .Hyaluronan constitutively regulates ErbB2 phosphorylation and signaling complex formation in carcinoma cells[J].J Biol Chem,2005,280(10):8875-8883.
doi: 10.1074/jbc.M410882200 |
| 28 |
HuangW , YeM , ZhangLR ,et al.FW-04-806 inhibits proliferation and induces apoptosis in human breast cancer cells by binding to N-terminus of Hsp90 and disrupting Hsp90-Cdc37 complex formation[J].Mol Cancer,2014,13,150.
doi: 10.1186/1476-4598-13-150 |
| 29 |
Esparís-OgandoA , MonteroJC , ArribasJ ,et al.Targeting the EGF/HER ligand-receptor system in cancer[J].Curr Pharm Des,2016,22(39):5887-5898.
doi: 10.2174/1381612822666160715132233 |
| 30 | WangZ .ErbB receptors and cancer[J].Methods Mol Biol,2017,1652,3-35. |
| 31 | GarousiS , Jahanbakhsh-GodehkahrizS , EsfahaniK ,et al.Meta-analysis of EGF-stimulated normal and cancer cell lines to discover EGF-associated oncogenic signaling pathways and prognostic biomarkers[J].Iran J Biotechnol,2022,20(3):e3245. |
| 32 | LaczmanskaI , SasiadekMM .Tyrosine phosphatases as a superfamily of tumor suppressors in colorectal cancer[J].Acta Biochim Pol,2011,58(4):467-470. |
| 33 |
MenigattiM , CattaneoE , Sabates-BellverJ ,et al.The protein tyrosine phosphatase receptor type R gene is an early and frequent target of silencing in human colorectal tumorigenesis[J].Mol Cancer,2009,8,124.
doi: 10.1186/1476-4598-8-124 |
| 34 |
WangY , CaoJ , LiuW ,et al.Protein tyrosine phosphatase receptor type R (PTPRR) antagonizes the wnt signaling pathway in ovarian cancer by dephosphorylating and inactivating β-catenin[J].J Biol Chem,2019,294(48):18306-18323.
doi: 10.1074/jbc.RA119.010348 |
| 35 |
SuPH , LinYW , HuangRL ,et al.Epigenetic silencing of PTPRR activates MAPK signaling, promotes metastasis and serves as a biomarker of invasive cervical cancer[J].Oncogene,2013,32(1):15-26.
doi: 10.1038/onc.2012.29 |
| 36 |
MunkleyJ , LaffertyNP , KalnaG ,et al.Androgen-regulation of the protein tyrosine phosphatase PTPRR activates ERK1/2 signalling in prostate cancer cells[J].BMC Cancer,2015,15,9.
doi: 10.1186/s12885-015-1012-8 |
| 37 |
ChengWL , FengPH , LeeKY ,et al.The role of EREG/EGFR pathway in tumor progression[J].Int J Mol Sci,2021,22(23):12828.
doi: 10.3390/ijms222312828 |
| 38 |
ZhangL , NanF , YangL ,et al.Differentially expressed EREG and SPP1 are independent prognostic markers in cervical squamous cell carcinoma[J].J Obstet Gynaecol Res,2022,48(7):1848-1858.
doi: 10.1111/jog.15265 |
| 39 |
LiuS , WangY , HanY ,et al.EREG-driven oncogenesis of head and neck squamous cell carcinoma exhibits higher sensitivity to erlotinib therapy[J].Theranostics,2020,10(23):10589-10605.
doi: 10.7150/thno.47176 |
| 40 |
XiaQ , ZhouY , YongH ,et al.Elevated epiregulin expression predicts poor prognosis in gastric cancer[J].Pathol Res Pract,2019,215(5):873-879.
doi: 10.1016/j.prp.2019.01.030 |
| [1] | Junyong OU,Kunming NI,Lulin MA,Guoliang WANG,Ye YAN,Bin YANG,Gengwu LI,Haodong SONG,Min LU,Jianfei YE,Shudong ZHANG. Prognostic factors of patients with muscle invasive bladder cancer with intermediate-to-high risk prostate cancer [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 582-588. |
| [2] | Shuai LIU,Lei LIU,Zhuo LIU,Fan ZHANG,Lulin MA,Xiaojun TIAN,Xiaofei HOU,Guoliang WANG,Lei ZHAO,Shudong ZHANG. Clinical treatment and prognosis of adrenocortical carcinoma with venous tumor thrombus [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 624-630. |
| [3] | Le YU,Shaohui DENG,Fan ZHANG,Ye YAN,Jianfei YE,Shudong ZHANG. Clinicopathological characteristics and prognosis of multilocular cystic renal neoplasm of low malignant potential [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 661-666. |
| [4] | Zezhen ZHOU,Shaohui DENG,Ye YAN,Fan ZHANG,Yichang HAO,Liyuan GE,Hongxian ZHANG,Guoliang WANG,Shudong ZHANG. Predicting the 3-year tumor-specific survival in patients with T3a non-metastatic renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 673-679. |
| [5] | Yangyi FANG,Qiang LI,Zhigao HUANG,Min LU,Kai HONG,Shudong ZHANG. Well-differentiated papillary mesothelial tumour of the tunica vaginalis: A case report [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 741-744. |
| [6] | Zuoxiang LIU,Xiaowei CHEN,Houyu ZHAO,Siyan ZHAN,Feng SUN. Cardiovascular safety of sitagliptin added to metformin in real world patients with type 2 diabetes [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 424-430. |
| [7] | Yuanyuan ZENG,Yun XIE,Daonan CHEN,Ruilan WANG. Related factors of euthyroid sick syndrome in patients with sepsis [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 526-532. |
| [8] | Jian-bin LI,Meng-na LYU,Qiang CHI,Yi-lin PENG,Peng-cheng LIU,Rui WU. Early prediction of severe COVID-19 in patients with Sjögren’s syndrome [J]. Journal of Peking University (Health Sciences), 2023, 55(6): 1007-1012. |
| [9] | Zi-xuan XUE,Shi-ying TANG,Min QIU,Cheng LIU,Xiao-jun TIAN,Min LU,Jing-han DONG,Lu-lin MA,Shu-dong ZHANG. Clinicopathologic features and prognosis of young renal tumors with tumor thrombus [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 802-811. |
| [10] | Han LU,Jian-yun ZHANG,Rong YANG,Le XU,Qing-xiang LI,Yu-xing GUO,Chuan-bin GUO. Clinical factors affecting the prognosis of lower gingival squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 702-707. |
| [11] | Yun-fei SHI,Hao-jie WANG,Wei-ping LIU,Lan MI,Meng-ping LONG,Yan-fei LIU,Yu-mei LAI,Li-xin ZHOU,Xin-ting DIAO,Xiang-hong LI. Analysis of clinicopathological and molecular abnormalities of angioimmunoblastic T-cell lymphoma [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 521-529. |
| [12] | Xiao-juan ZHU,Hong ZHANG,Shuang ZHANG,Dong LI,Xin LI,Ling XU,Ting LI. Clinicopathological features and prognosis of breast cancer with human epidermal growth factor receptor 2 low expression [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 243-253. |
| [13] | Yu-mei LAI,Zhong-wu LI,Huan LI,Yan WU,Yun-fei SHI,Li-xin ZHOU,Yu-tong LOU,Chuan-liang CUI. Clinicopathological features and prognosis of anorectal melanoma: A report of 68 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 262-269. |
| [14] | Qi SHEN,Yi-xiao LIU,Qun HE. Mucinous tubular and spindle cell carcinoma of kidney: Clinicopathology and prognosis [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 276-282. |
| [15] | Qian SU,Xin PENG,Chuan-xiang ZHOU,Guang-yan YU. Clinicopathological characteristics and prognosis of non-Hodgkin lymphoma in oral and maxillofacial regions: An analysis of 369 cases [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 13-21. |
|
||