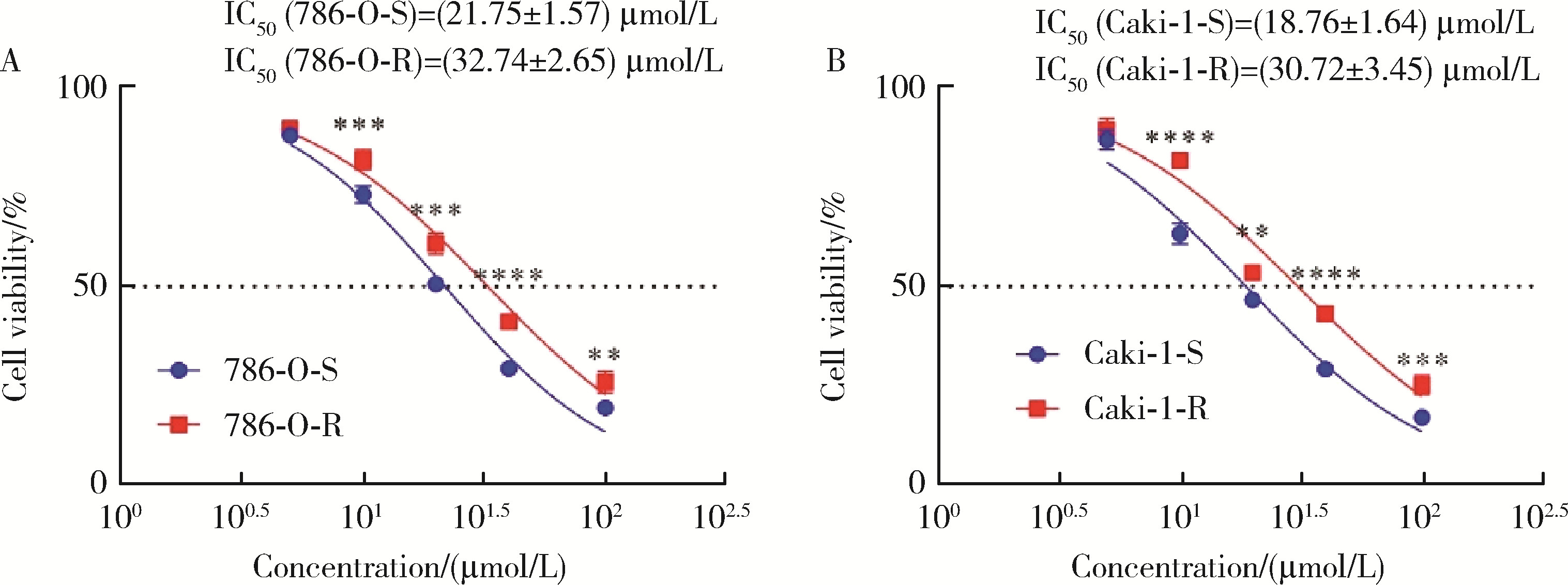
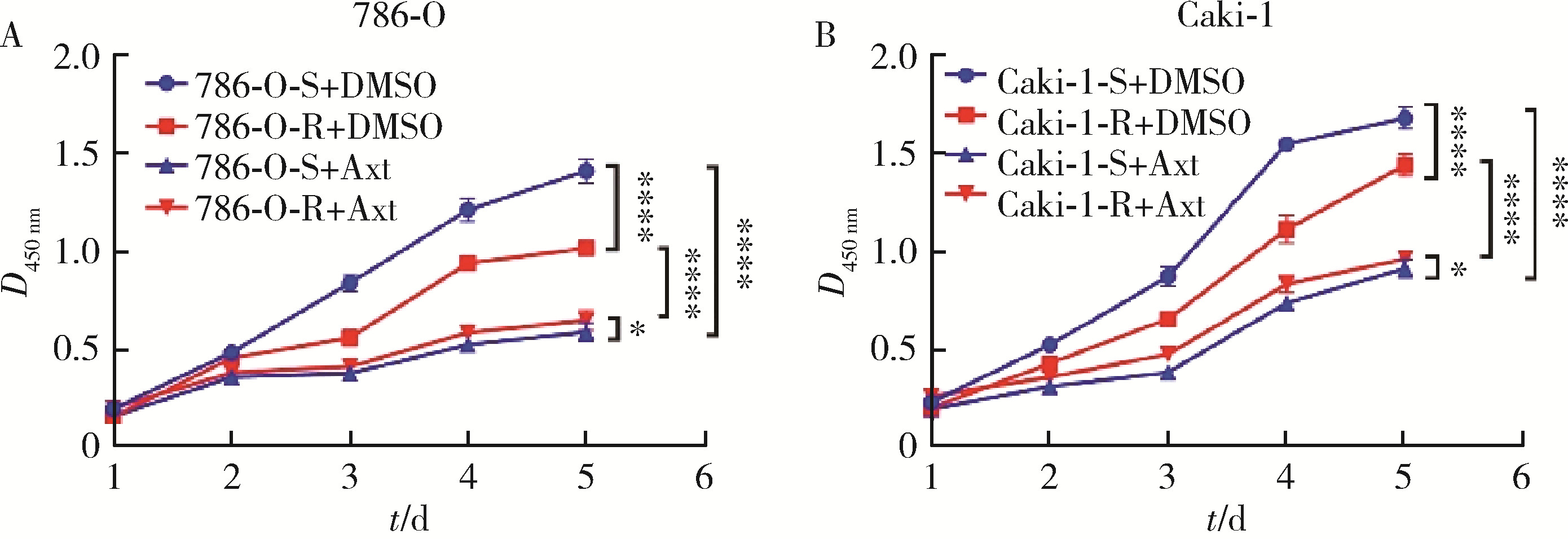
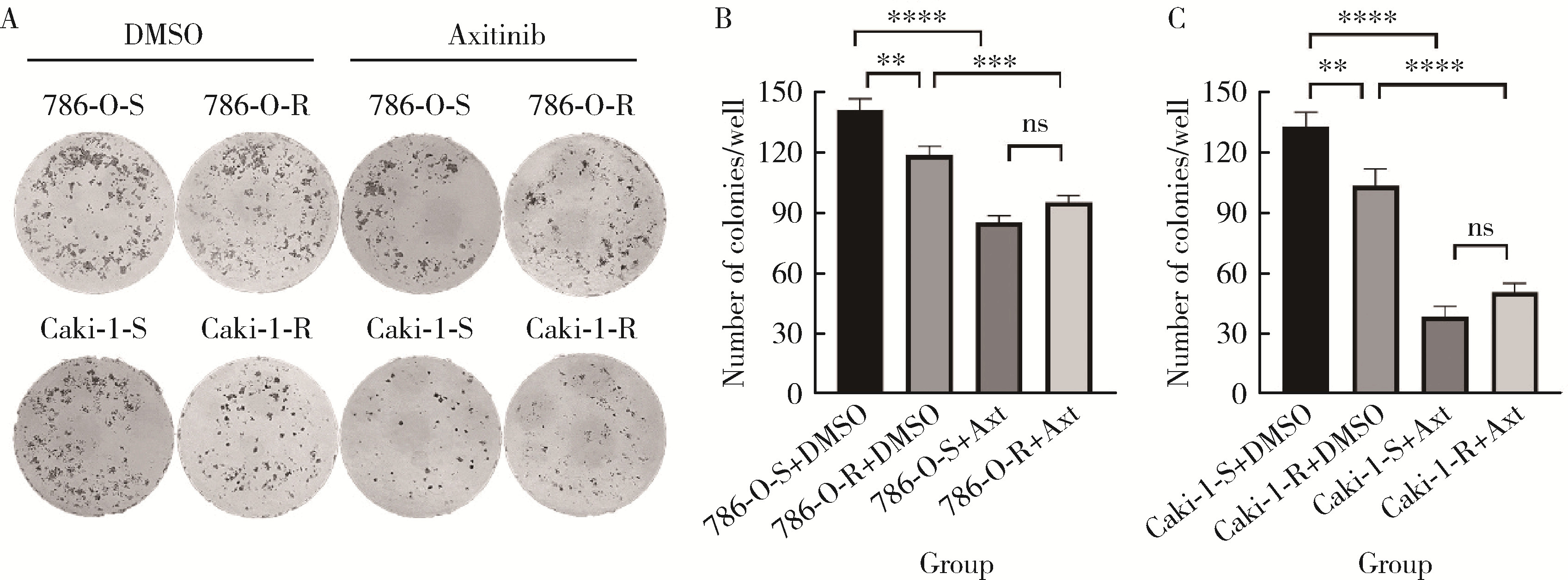
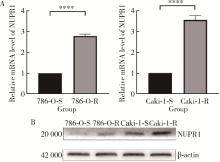
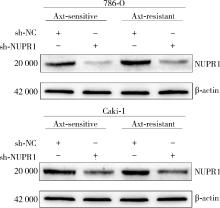
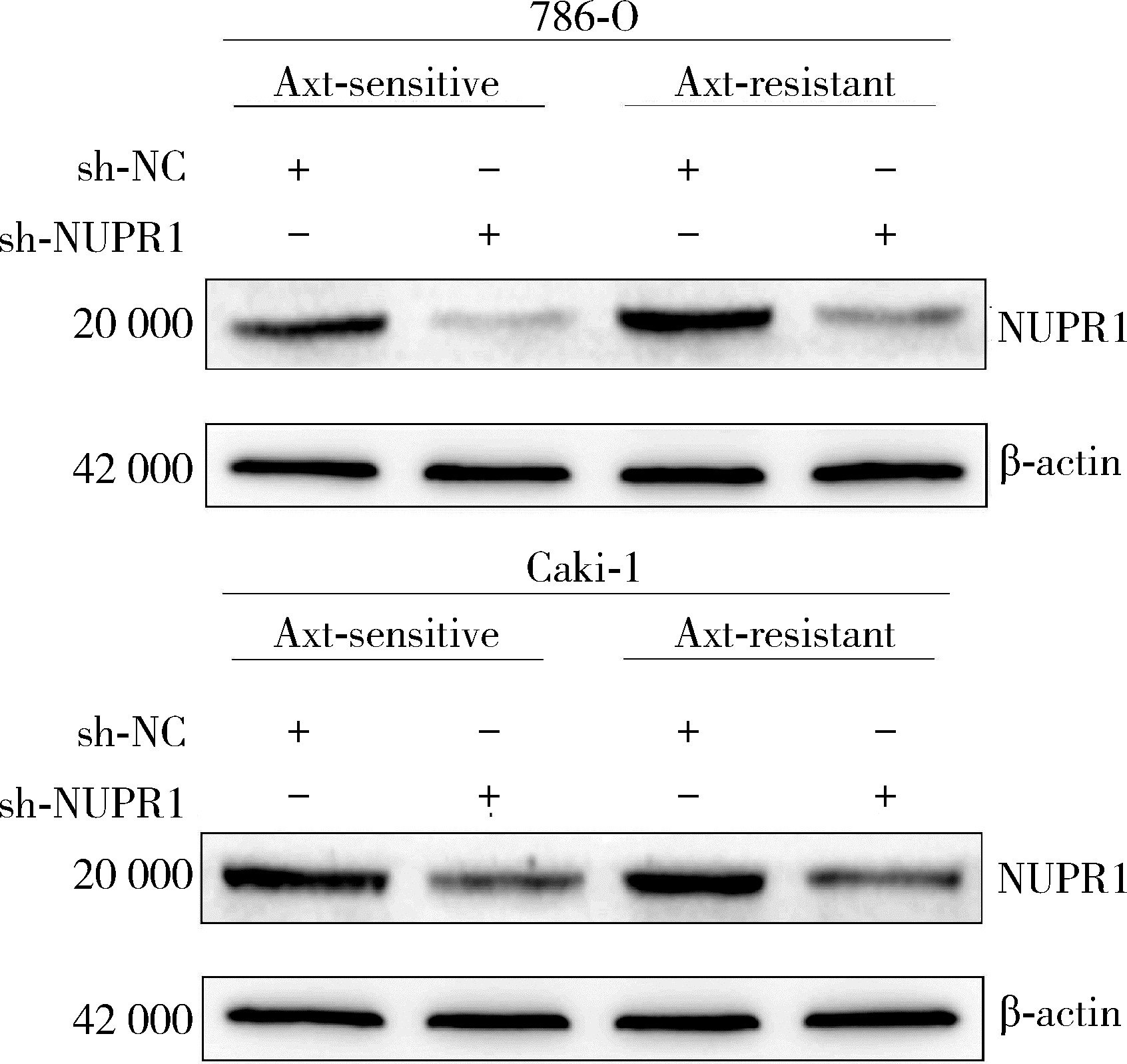
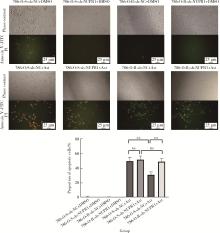
Journal of Peking University (Health Sciences) ›› 2023, Vol. 55 ›› Issue (5): 781-792. doi: 10.19723/j.issn.1671-167X.2023.05.003
Previous Articles Next Articles
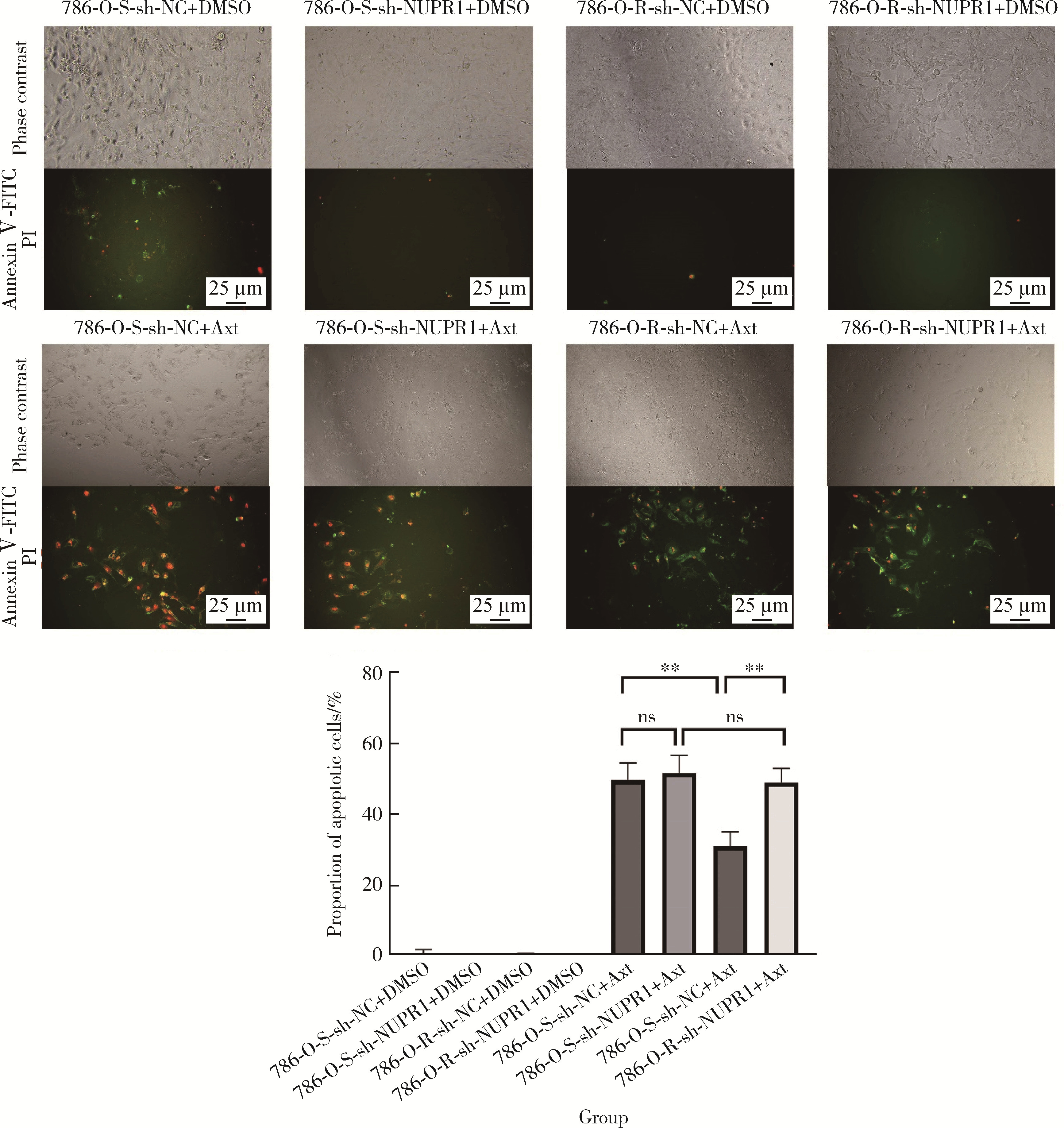
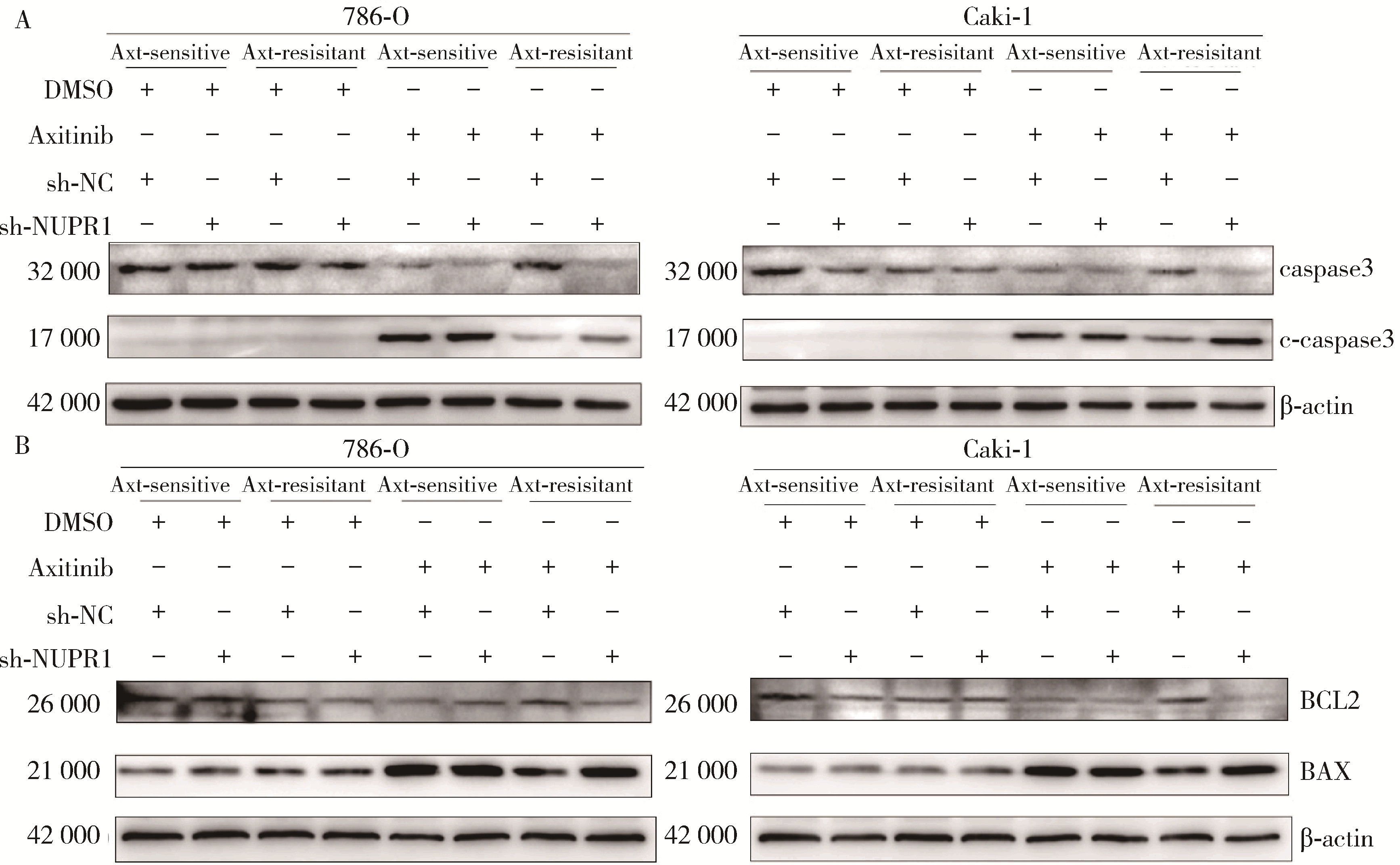
Mechanism of nuclear protein 1 in the resistance to axitinib in clear cell renal cell carcinoma
Yun-chong LIU,Zong-long WU,Li-yuan GE,Tan DU,Ya-qian WU,Yi-meng SONG,Cheng LIU*( ),Lu-lin MA*(
),Lu-lin MA*( )
)
- Department of Urology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R737.1
| 1 |
SiegelRL , MillerKD , WagleNS ,et al.Cancer statistics, 2023[J].CA Cancer J Clin,2023,73(1):17-48.
doi: 10.3322/caac.21763 |
| 2 |
MotzerRJ , BukowskiRM , FiglinRA ,et al.Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma[J].Cancer,2008,113(7):1552-1558.
doi: 10.1002/cncr.23776 |
| 3 | NerichV , HuguesM , PaillardMJ ,et al.Clinical impact of targeted therapies in patients with metastatic clear-cell renal cell carcinoma[J].Onco Targets Ther,2014,7,365-374. |
| 4 |
PowlesT , PlimackER , SoulièresD ,et al.Pembrolizumab plus axitinib versus sunitinib monotherapy as first-line treatment of advanced renal cell carcinoma (KEYNOTE-426): Extended follow-up from a randomised, open-label, phase 3 trial[J].Lancet Oncol,2020,21(12):1563-1573.
doi: 10.1016/S1470-2045(20)30436-8 |
| 5 |
IncorvaiaL , MadoniaG , CorsiniLR ,et al.Challenges and advances for the treatment of renal cancer patients with brain metastases: From immunological background to upcoming clinical evidence on immune-checkpoint inhibitors[J].Crit Rev Oncol Hematol,2021,163,103390.
doi: 10.1016/j.critrevonc.2021.103390 |
| 6 |
ShepardDR , GarciaJA .Toxicity associated with the long-term use of targeted therapies in patients with advanced renal cell carcinoma[J].Expert Rev Anticancer Ther,2009,9(6):795-805.
doi: 10.1586/era.09.29 |
| 7 |
RiniBI , EscudierB , TomczakP ,et al.Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): A randomised phase 3 trial[J].Lancet,2011,378(9807):1931-1939.
doi: 10.1016/S0140-6736(11)61613-9 |
| 8 | CaiW , CaiB , ZhouJ ,et al.Comparison of efficacy and safety among axitinib, sunitinib, and sorafenib as neoadjuvant therapy for renal cell carcinoma: A retrospective study[J].Cancer Commun (Lond),2019,39(1):56. |
| 9 |
ZhouX , HouW , GaoL ,et al.Synergies of antiangiogenic therapy and immune checkpoint blockade in renal cell carcinoma: From theoretical background to clinical reality[J].Front Oncol,2020,10,1321.
doi: 10.3389/fonc.2020.01321 |
| 10 |
GulatiS , LabakiC , KarachaliouGS ,et al.First-line treatments for metastatic clear cell renal cell carcinoma: An ever-enlarging landscape[J].Oncologist,2022,27(2):125-134.
doi: 10.1093/oncolo/oyab056 |
| 11 |
ChoueiriTK , MotzerRJ , RiniBI ,et al.Updated efficacy results from the JAVELIN Renal 101 trial: First-line avelumab plus axitinib versus sunitinib in patients with advanced renal cell carcinoma[J].Ann Oncol,2020,31(8):1030-1039.
doi: 10.1016/j.annonc.2020.04.010 |
| 12 |
BuchlerT , BortlicekZ , PoprachA ,et al.Outcomes for patients with metastatic renal cell carcinoma achieving a complete response on targeted therapy: A registry-based analysis[J].Eur Urol,2016,70(3):469-475.
doi: 10.1016/j.eururo.2015.12.031 |
| 13 |
BuchlerT , PoprachA , BortlicekZ ,et al.Outcomes of patients with long-term treatment response to vascular endothelial growth factor-targeted therapy for metastatic renal cell cancer[J].Clin Genitourin Cancer,2017,15(6):e1047-e1053.
doi: 10.1016/j.clgc.2017.06.006 |
| 14 |
LuS , SteinJE , RimmDL ,et al.Comparison of biomarker moda-lities for predicting response to PD-1/PD-L1 checkpoint blockade: A systematic review and meta-analysis[J].JAMA Oncol,2019,5(8):1195-1204.
doi: 10.1001/jamaoncol.2019.1549 |
| 15 |
BallesterosP , ChamorroJ , Román-GilMS ,et al.Molecular mechanisms of resistance to immunotherapy and antiangiogenic treatments in clear cell renal cell carcinoma[J].Cancers (Basel),2021,13(23):5981.
doi: 10.3390/cancers13235981 |
| 16 |
XiangY , ZhengG , ZhongJ ,et al.Advances in renal cell carcinoma drug resistance models[J].Front Oncol,2022,12,870396.
doi: 10.3389/fonc.2022.870396 |
| 17 | HeW , ChengF , ZhengB ,et al.NUPR1 is a novel potential biomarker and confers resistance to sorafenib in clear cell renal cell carcinoma by increasing stemness and targeting the PTEN/AKT/mTOR pathway[J].Aging (Albany NY),2021,13(10):14015-14038. |
| 18 |
GotinkKJ , VerheulHM .Anti-angiogenic tyrosine kinase inhibitors: What is their mechanism of action?[J].Angiogenesis,2010,13(1):1-14.
doi: 10.1007/s10456-009-9160-6 |
| 19 |
EllisLM , HicklinDJ .VEGF-targeted therapy: Mechanisms of anti-tumour activity[J].Nat Rev Cancer,2008,8(8):579-591.
doi: 10.1038/nrc2403 |
| 20 |
ChenY , LuZ , QiC ,et al.N(6)-methyladenosine-modified TRAF1 promotes sunitinib resistance by regulating apoptosis and angiogenesis in a METTL14-dependent manner in renal cell carcinoma[J].Mol Cancer,2022,21(1):111.
doi: 10.1186/s12943-022-01549-1 |
| 21 | SakaiI , MiyakeH , FujisawaM .Acquired resistance to sunitini-bin human renal cell carcinoma cells is mediated by constitutive activation of signal transduction pathways associated with tumour cell proliferation[J].BJU Int,2013,112(2):211-220. |
| 22 |
AzamF , MehtaS , HarrisAL .Mechanisms of resistance to antiangiogenesis therapy[J].Eur J Cancer,2010,46(8):1323-1332.
doi: 10.1016/j.ejca.2010.02.020 |
| 23 |
HuangH , ZhangJ , JiangP ,et al.FXR1 facilitates axitinib resistance in clear cell renal cell carcinoma via regulating KEAP1/Nrf2 signaling pathway[J].Anticancer Drugs,2023,34(2):248-256.
doi: 10.1097/CAD.0000000000001416 |
| 24 |
MalloGV , FiedlerF , CalvoEL ,et al.Cloning and expression of the rat p8 cDNA, a new gene activated in pancreas during the acute phase of pancreatitis, pancreatic development, and regeneration, and which promotes cellular growth[J].J Biol Chem,1997,272(51):32360-32369.
doi: 10.1074/jbc.272.51.32360 |
| 25 |
EmmaMR , IovannaJL , BachvarovD ,et al.NUPR1, a new target in liver cancer: implication in controlling cell growth, migration, invasion and sorafenib resistance[J].Cell Death Dis,2016,7(6):e2269.
doi: 10.1038/cddis.2016.175 |
| 26 |
MartinTA , LiAX , SandersAJ ,et al.NUPR1 and its potential role in cancer and pathological conditions[J].Int J Oncol,2021,58(5):21.
doi: 10.3892/ijo.2021.5201 |
| 27 |
Santofimia-CastañoP , LanW , BintzJ ,et al.Inactivation of NUPR1 promotes cell death by coupling ER-stress responses with necrosis[J].Sci Rep,2018,8(1):16999.
doi: 10.1038/s41598-018-35020-3 |
| 28 |
MaddenE , LogueSE , HealySJ ,et al.The role of the unfolded protein response in cancer progression: From oncogenesis to chemoresistance[J].Biol Cell,2019,111(1):1-17.
doi: 10.1111/boc.201800050 |
| 29 |
HetzC .The unfolded protein response: Controlling cell fate decisions under ER stress and beyond[J].Nat Rev Mol Cell Biol,2012,13(2):89-102.
doi: 10.1038/nrm3270 |
| 30 |
KowalczykT , SitarekP , SkałaE ,et al.Induction of apoptosis by in vitro and in vivo plant extracts derived from Menyanthes trifoliata L. in human cancer cells[J].Cytotechnology,2019,71(1):165-180.
doi: 10.1007/s10616-018-0274-9 |
| 31 |
KluckRM , Bossy-WetzelE , GreenDR ,et al.The release of cytochrome c from mitochondria: A primary site for Bcl-2 regulation of apoptosis[J].Science,1997,275(5303):1132-1136.
doi: 10.1126/science.275.5303.1132 |
| [1] | Tian-yu CAI,Zhen-peng ZHU,Chun-ru XU,Xing JI,Tong-de LV,Zhen-ke GUO,Jian LIN. Expression and significance of fibroblast growth factor receptor 2 in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 628-635. |
| [2] | Mei-ni ZUO,Yi-qing DU,Lu-ping YU,Xiang DAI,Tao XU. Correlation between metabolic syndrome and prognosis of patients with clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 636-643. |
| [3] | SU Jun-qi,SONG Yang,XIE Shang. Analysis of etiological characteristics and establishment of prediction model of postoperative infections in patients undergoing oral squamous cell carcinoma surgery with free flap reconstruction [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 68-76. |
| [4] | GAO Ya-dong,ZHU An,LI Lu-di,ZHANG Tao,WANG Shuo,SHAN Dan-ping,LI Ying-zi,WANG Qi. Cytotoxicity and underlying mechanism of evodiamine in HepG2 cells [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1107-1114. |
| [5] | LOU Xue,LIAO Li,LI Xing-jun,WANG Nan,LIU Shuang,CUI Ruo-mei,XU Jian. Methylation status and expression of TWEAK gene promoter region in peripheral blood of patients with rheumatoid arthritis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1020-1025. |
| [6] | Lei-zhen SU,Jie CHEN,Xian LI,Ping JI. Effects of salinomycin on proliferation and apoptosis of oral squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 902-906. |
| [7] | Liang GENG,Jing LV,Jing FAN. Effect of Fei-Liu-Ping ointment combined with cyclophosphamide on lung cancer cell proliferation and acidic microenvironment [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 247-253. |
| [8] | Si-mei REN,Lu-yao LONG,Cheng-shan XU. Interaction between PSF and cytokeratin 18 mediates PSF relocation to cell membrane and maintains chemosensitivity of myeloid leukemia [J]. Journal of Peking University (Health Sciences), 2020, 52(2): 214-220. |
| [9] | LI Man, LI Yuan, SUN Lin, SONG Jun-lai, LV Cong. High mobility group box 1 promotes apoptosis of astrocytes after oxygen glucose deprivation/reoxygenation by regulating the expression of Bcl-2 and Bax [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 785-791. |
| [10] | SUN Jing, SONG Wei-dong, YAN Si-yuan, XI Zhi-jun. Chloroquine inhibits viability of renal carcinoma cells and enhances sunitinib-induced caspase-dependent apoptosis [J]. Journal of Peking University(Health Sciences), 2018, 50(5): 778-784. |
| [11] | WANG Hao, CHEN Liang, YE Xiao-yun. Triptolide induces oxidative stress and apoptosis and activates PIK3/Akt signaling pathway in TM4 sertoli cells [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 607-612. |
| [12] | WANG Yu-jie, GUO Xiang-yang, WANG Jun. Influences of repeated propofol anesthesia on hippocampal apoptosis and long-term learning and memory abilities of neonatal rats [J]. Journal of Peking University(Health Sciences), 2017, 49(2): 310-314. |
| [13] | YANG Guang, CHENG Qing-li, LI Chun-lin, JIA Ya-li, YUE Wen, PEI Xue-tao, LIU Yang, ZHAO Jia-hui, DU Jing, AO Qiang-guo. High glucose reduced the repair function of kidney stem cells conditional medium to the hypoxia-injured renal tubular epithelium cells [J]. Journal of Peking University(Health Sciences), 2017, 49(1): 125-130. |
| [14] | CAO Pei, JIANG Xue-jun, XI Zhi-jun. Sunitinib induces autophagy via suppressing Akt/mTOR pathway in renal cell carcinoma [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 584-589. |
| [15] | LI Gang, ZHANG Hong-xian, WANG Yun-peng, ZHANG Jing,HONG Kai, TIAN Xiao-jun, MA Lu-lin. Protective effect of phloroglucinol on renal ischemia and reperfusion injury [J]. Journal of Peking University(Health Sciences), 2015, 47(5): 743-748. |
|
||