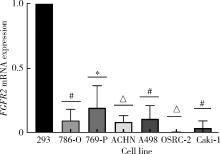
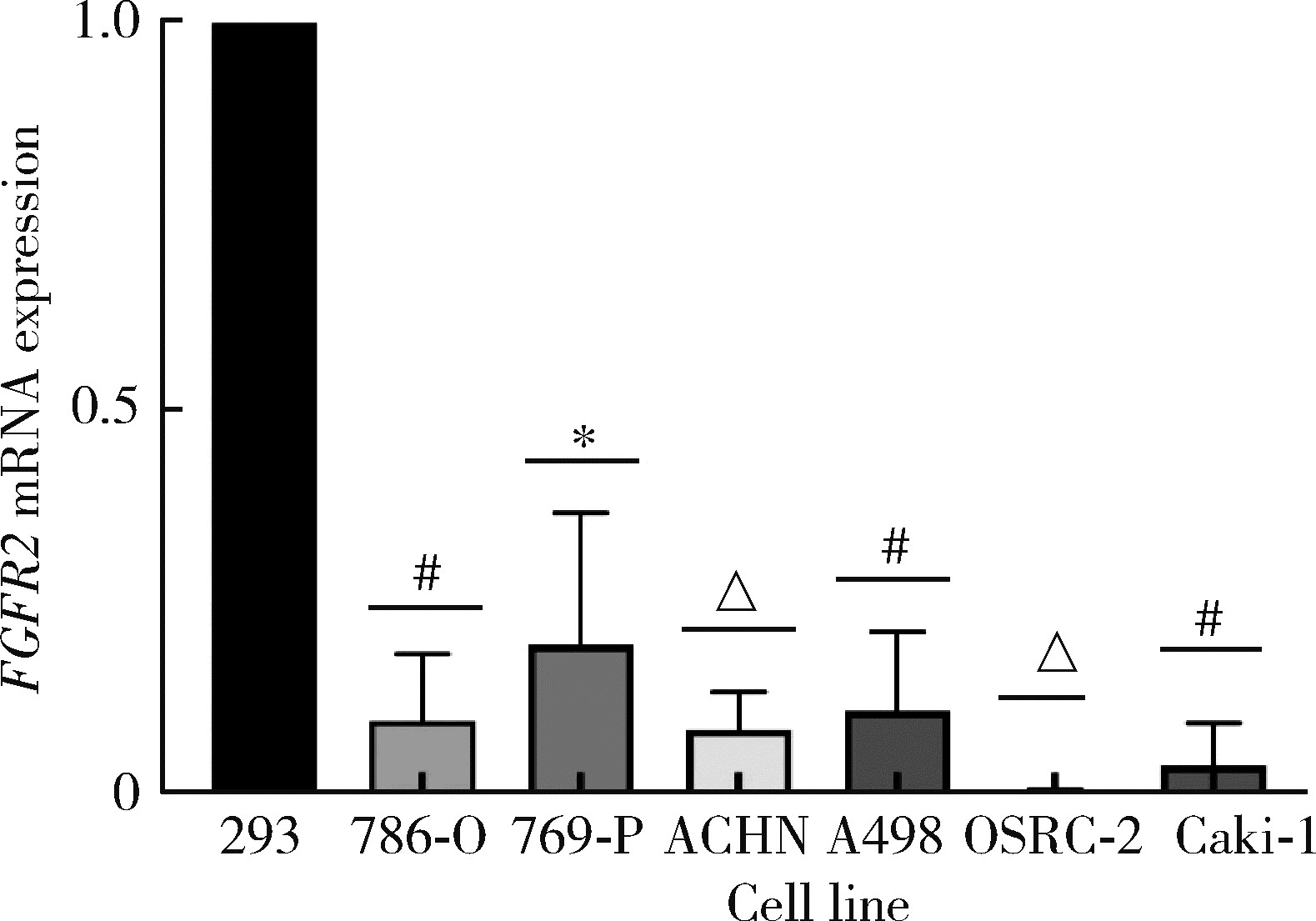
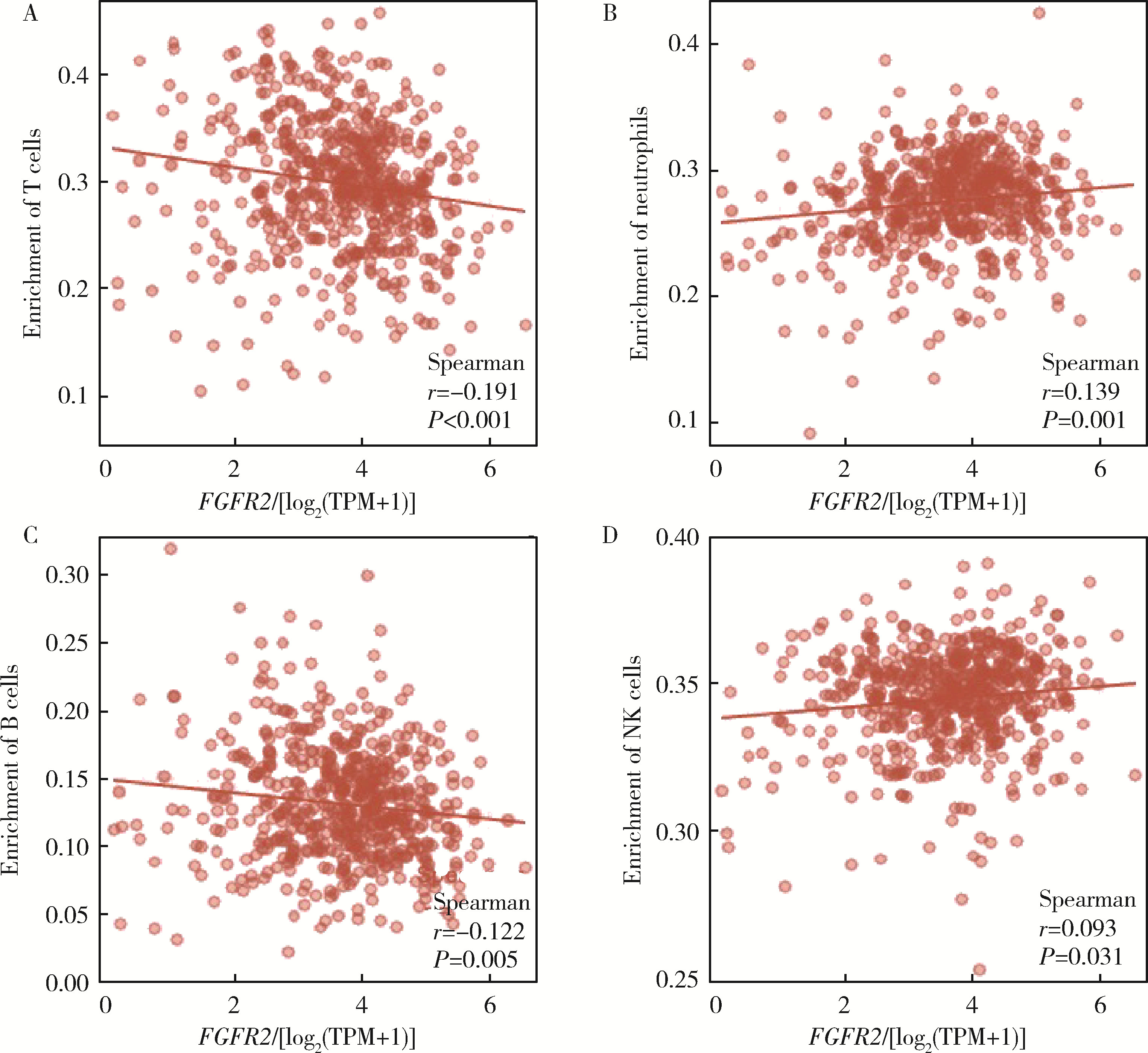
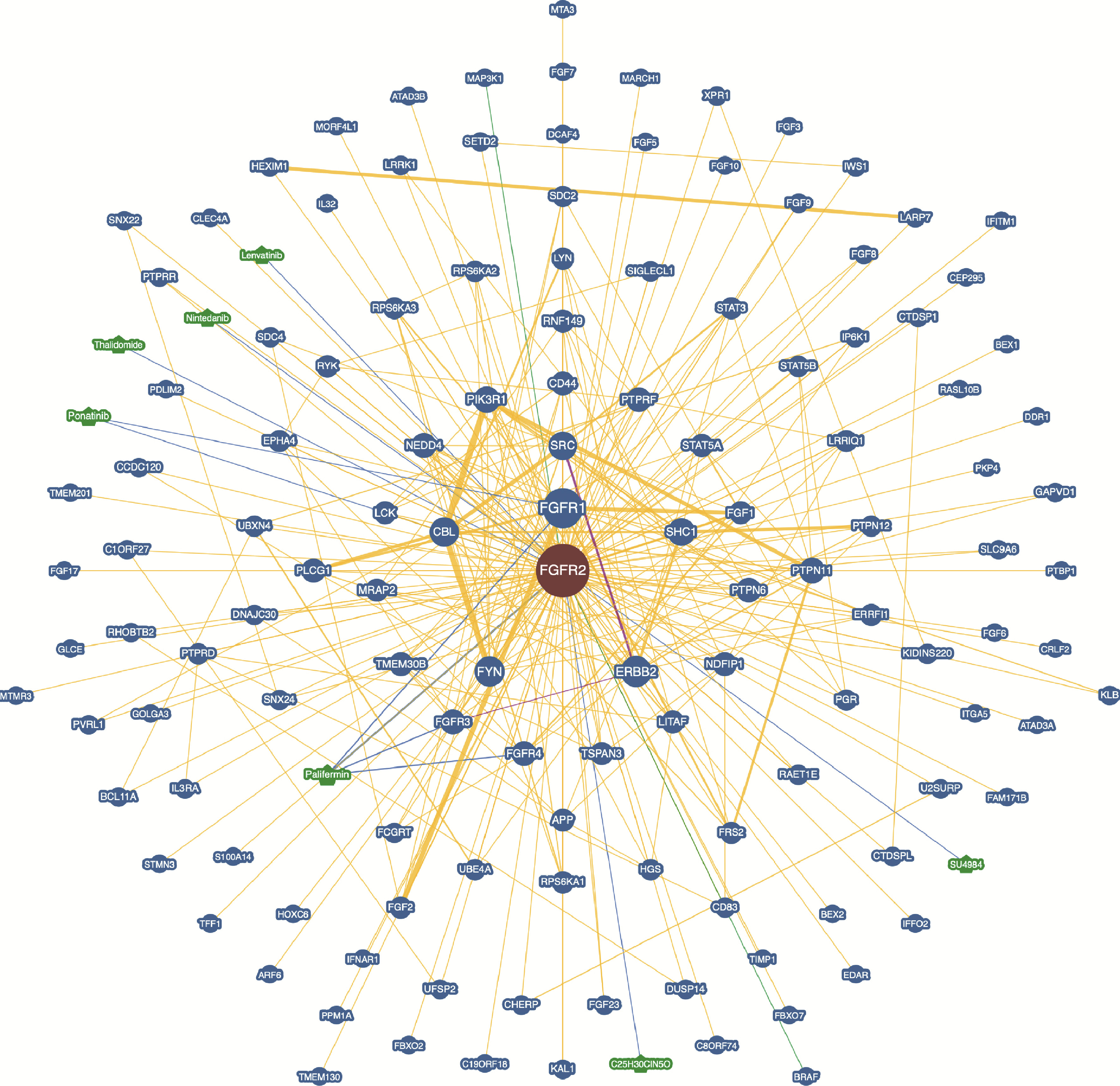
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (4): 628-635. doi: 10.19723/j.issn.1671-167X.2022.04.008
Previous Articles Next Articles
Expression and significance of fibroblast growth factor receptor 2 in clear cell renal cell carcinoma
Tian-yu CAI,Zhen-peng ZHU,Chun-ru XU,Xing JI,Tong-de LV,Zhen-ke GUO,Jian LIN*( )
)
- Department of Urology, Peking University First Hospital; Institute of Urology, Peking University; National Urological Can-cer Center, Beijing 100034, China
CLC Number:
- R34
| 1 |
Cohen HT . Renal-cell carcinoma[J]. N Engl J Med, 2005, 353 (23): 2477- 2490.
doi: 10.1056/NEJMra043172 |
| 2 |
Siegel RL , Miller KD , Fuchs HE , et al. Cancer statistics, 2021[J]. CA Cancer J Clin, 2021, 71 (1): 7- 33.
doi: 10.3322/caac.21654 |
| 3 |
Hsieh JJ , Purdue MP , Signoretti S , et al. Renal cell carcinoma[J]. Nat Rev Dis Primers, 2017, 3 (1): 17009.
doi: 10.1038/nrdp.2017.9 |
| 4 |
Rini BI , Campbell SC , Escudier B . Renal cell carcinoma[J]. Lancet, 2009, 373 (9669): 1119- 1132.
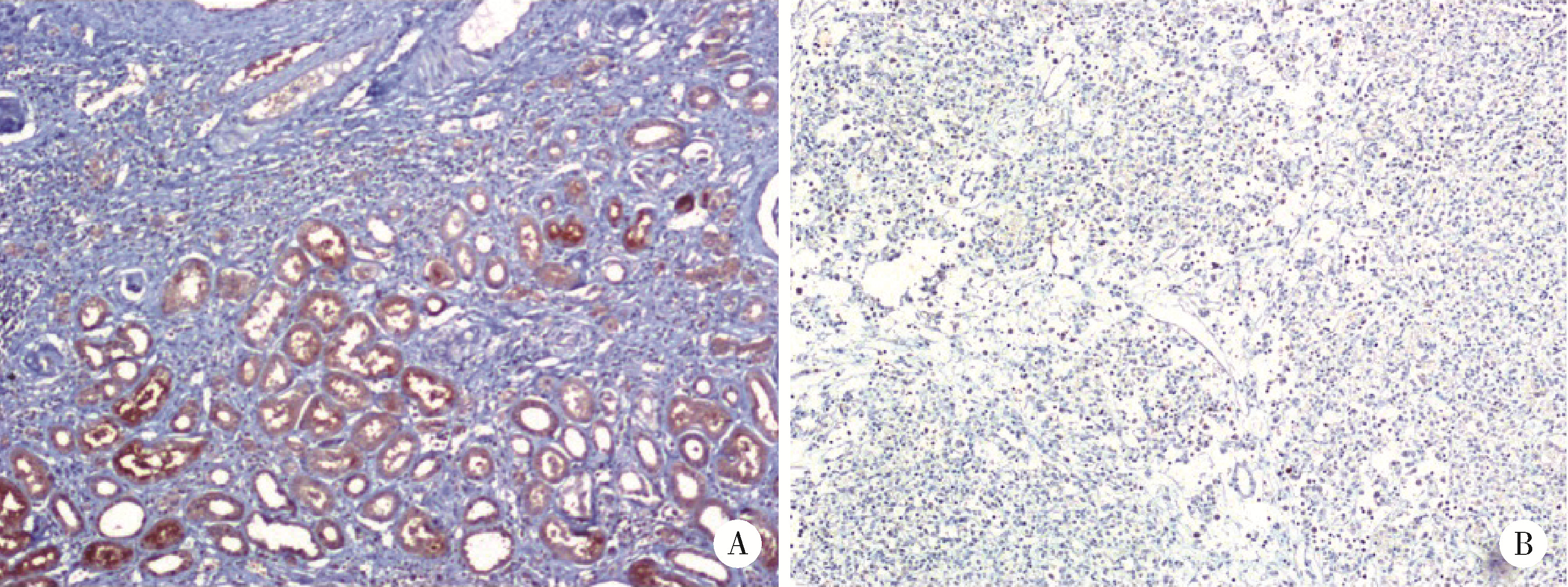
doi: 10.1016/S0140-6736(09)60229-4 |
| 5 | Penticuff JC , Kyprianou N . Therapeutic challenges in renal cell carcinoma[J]. Am J Clin Exp Urol, 2015, 3 (2): 77- 90. |
| 6 |
Wolff I , May M , Hoschke B , et al. Do we need new high-risk criteria for surgically treated renal cancer patients to improve the outcome of future clinical trials in the adjuvant setting? Results of a comprehensive analysis based on the multicenter CORONA database[J]. Eur J Surg Oncol, 2016, 42 (5): 744- 750.
doi: 10.1016/j.ejso.2016.01.009 |
| 7 |
Williamson TJ , Pearson JR , Ischia J , et al. Guideline of guidelines: follow-up after nephrectomy for renal cell carcinoma[J]. BJU Int, 2016, 117 (4): 555- 562.
doi: 10.1111/bju.13384 |
| 8 |
Eswarakumar VP , Lax I , Schlessinger J . Cellular signaling by fibroblast growth factor receptors[J]. Cytokine Growth Factor Rev, 2005, 16 (2): 139- 149.
doi: 10.1016/j.cytogfr.2005.01.001 |
| 9 |
Li P , Huang T , Zou Q , et al. FGFR2 promotes expression of PD-L1 in colorectal cancer via the JAK/STAT3 signaling pathway[J]. J Immunol, 2019, 202 (10): 3065- 3075.
doi: 10.4049/jimmunol.1801199 |
| 10 |
Wang Y , Shi T , Wang X , et al. FGFR2 alteration as a potential therapeutic target in poorly cohesive gastric carcinoma[J]. J Transl Med, 2021, 19 (1): 1- 11.
doi: 10.1186/s12967-020-02683-4 |
| 11 |
Oughtred R , Rust J , Chang C , et al. The BioGRID database: a comprehensive biomedical resource of curated protein, genetic, and chemical interactions[J]. Protein Sci, 2021, 30 (1): 187- 200.
doi: 10.1002/pro.3978 |
| 12 |
Siegel RL , Miller KD , Jemal A . Cancer statistics, 2020[J]. CA Cancer J Clin, 2020, 70 (1): 7- 30.
doi: 10.3322/caac.21590 |
| 13 | Turner N , Grose R . Fibroblast growth factor signalling: from development to cancer[J]. Nat Rev Cancer, 2010, 10 (2): 116- 129. |
| 14 |
Zhang J , Wong CC , Leung KT , et al. FGF18-FGFR2 signaling triggers the activation of c-Jun-YAP1 axis to promote carcinoge-nesis in a subgroup of gastric cancer patients and indicates translational potential[J]. Oncogene, 2020, 39 (43): 6647- 6663.
doi: 10.1038/s41388-020-01458-x |
| 15 |
Huang T , Liu D , Wang Y , et al. FGFR2 Promotes gastric cancer progression by inhibiting the expression of thrombospondin4 via PI3K-Akt-Mtor pathway[J]. Cell Physiol Biochem, 2018, 50 (4): 1332- 1345.
doi: 10.1159/000494590 |
| 16 |
Lei JH , Lee MH , Miao K , et al. Activation of FGFR2 signaling suppresses BRCA1 and drives triple-negative mammary tumorigenesis that is sensitive to immunotherapy[J]. Adv Sci, 2021, 8 (21): e2100974.
doi: 10.1002/advs.202100974 |
| 17 | Goyal L , Shi L , Liu LY , et al. TAS-120 overcomes resistance to ATP-competitive FGFR inhibitors in patients with FGFR2 fusion-positive intrahepatic cholangiocarcinoma[J]. Cancer Discov, 2019, 9 (8): 1064- 1079. |
| 18 |
Lee JE , Shin SH , Shin HW , et al. Nuclear FGFR2 negatively regulates hypoxia-induced cell invasion in prostate cancer by interacting with HIF-1 and HIF-2[J]. Sci Rep, 2019, 9 (1): 3480.
doi: 10.1038/s41598-019-39843-6 |
| 19 | Volkova M , Tsimafeyeu I , Olshanskaya A , et al. Immunochemical expression of fibroblast growth factor and its receptors in primary tumor cells of renal cell carcinoma[J]. Am J Clin Exp Urol, 2021, 9 (1): 65- 72. |
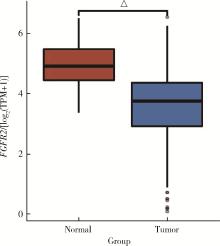
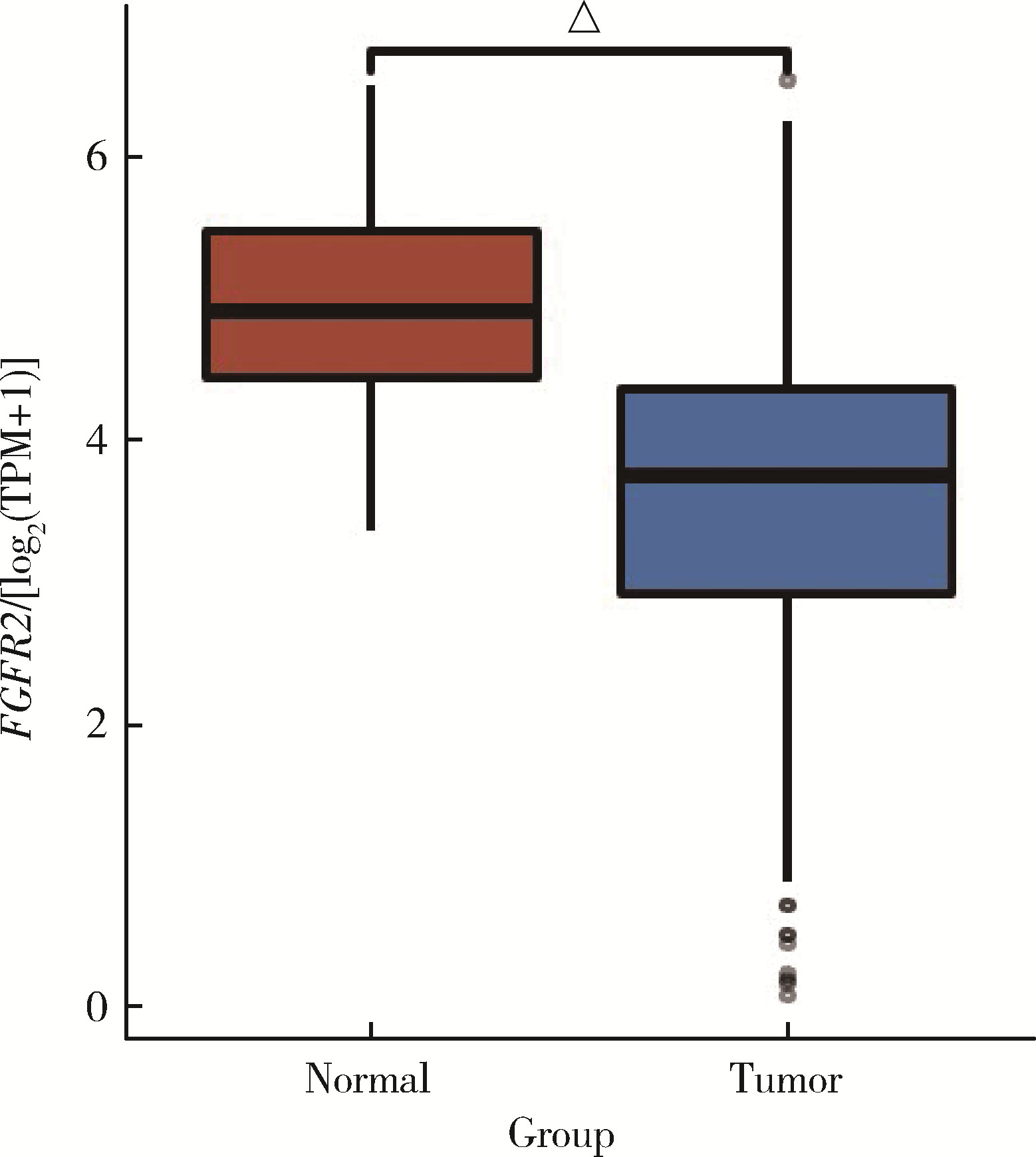
| 20 | Zhao Q , Caballero OL , Davis ID , et al. Tumor-specific isoform switch of the fibroblast growth factor receptor 2 underlies the mesenchymal and malignant phenotypes of clear cell renal cell carcinomas[J]. Clin Cancer Res, 2013, 19 (9): 2460- 2472. |
| 21 | Ranieri D , Nanni M , Guttieri L , et al. The aberrant expression in epithelial cells of the mesenchymal isoform of FGFR2 controls the negative crosstalk between EMT and autophagy[J]. J Cell Mol Med, 2021, 25 (8): 4166- 4172. |
| [1] | Yun-chong LIU,Zong-long WU,Li-yuan GE,Tan DU,Ya-qian WU,Yi-meng SONG,Cheng LIU,Lu-lin MA. Mechanism of nuclear protein 1 in the resistance to axitinib in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 781-792. |
| [2] | Zhong CAO,Hong-bing CEN,Jian-hong ZHAO,Jun MEI,Ling-zhi QIN,Wei LIAO,Qi-lin AO. Expression and significance of INSM1 and SOX11 in pancreatic neuroendocrine tumor and solid pseudopapillary neoplasm [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 575-581. |
| [3] | Fang CAO,Ming ZHONG,Cong-rong LIU. Uterine POLE mutant endometrioid carcinoma combined with human papilloma virus-associated cervical adenocarcinoma: A case report and literature review [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 370-374. |
| [4] | Li LIANG,Xin LI,Lin NONG,Ying DONG,Ji-xin ZHANG,Dong LI,Ting LI. Analysis of microsatellite instability in endometrial cancer: The significance of minimal microsatellite shift [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 254-261. |
| [5] | Mei-ni ZUO,Yi-qing DU,Lu-ping YU,Xiang DAI,Tao XU. Correlation between metabolic syndrome and prognosis of patients with clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 636-643. |
| [6] | PANG Yong,ZHANG Sha,YANG Hua,ZHOU Rou-li. Serum LAPTM4B-35 protein as a novel diagnostic marker for hepatocellular carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 710-715. |
| [7] | ZHOU Chuan, MA Xue, XING Yun-kun, LI Lu-di, CHEN Jie, YAO Bi-yun, FU Juan-ling, ZHAO Peng. Exploratory screening of potential pan-cancer biomarkers based on The Cancer Genome Atlas database [J]. Journal of Peking University (Health Sciences), 2021, 53(3): 602-607. |
| [8] | CHEN Huai-an,LIU Shuo,LI Xiu-jun,WANG Zhe,ZHANG Chao,LI Feng-qi,MIAO Wen-long. Clinical value of inflammatory biomarkers in predicting prognosis of patients with ureteral urothelial carcinoma [J]. Journal of Peking University (Health Sciences), 2021, 53(2): 302-307. |
| [9] | Xu-chu ZHANG,Jian-hua ZHANG,Rong-fu WANG,Yan FAN,Zhan-li FU,Ping YAN,Guang-yu ZHAO,Yan-xia BAI. Diagnostic value of 18F-FDG PET/CT and tumor markers (CEA, CA19-9, CA24-2) in recurrence and metastasis of postoperative colorectal moderately differentiated adenocarcinoma [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1071-1077. |
| [10] | Fei-long YANG,Kai HONG,Guo-jiang ZHAO,Cheng LIU,Yi-meng SONG,Lu-lin MA. Construction of prognostic model and identification of prognostic biomarkers based on the expression of long non-coding RNA in bladder cancer via bioinformatics [J]. Journal of Peking University(Health Sciences), 2019, 51(4): 615-622. |
| [11] | LIU Jin, XIONG Geng-yan, TANG Qi, FANG Dong, LI Xue-song, ZHOU Li-qun. Methylation status of RASSF1A gene promoter in upper tract urothelial carcinoma and its clinical significance [J]. Journal of Peking University(Health Sciences), 2016, 48(4): 571-578. |
|
||