Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (4): 636-643. doi: 10.19723/j.issn.1671-167X.2022.04.009
Previous Articles Next Articles
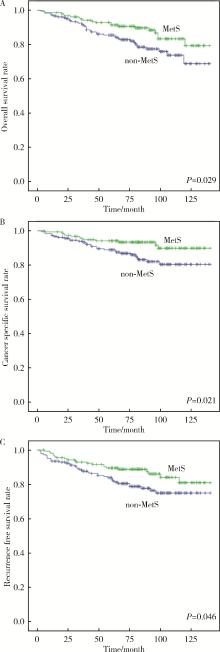
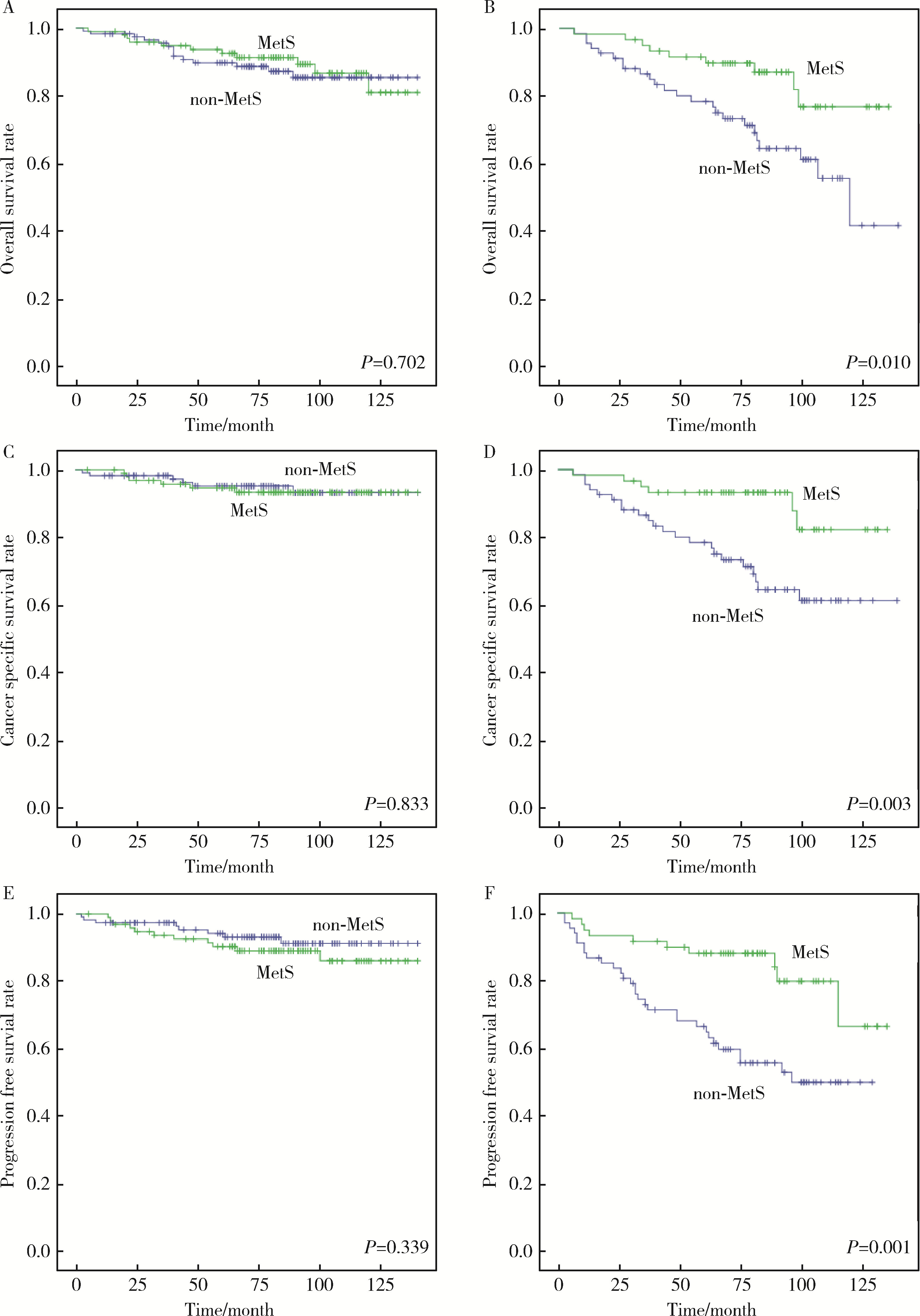
Correlation between metabolic syndrome and prognosis of patients with clear cell renal cell carcinoma
Mei-ni ZUO,Yi-qing DU,Lu-ping YU,Xiang DAI,Tao XU*( )
)
- Department of Urology, Peking University People's Hospital, Beijing 100044, China
CLC Number:
- R737
| 1 |
Bray F , Ferlay J , Soerjomataram I , et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries[J]. CA Cancer J Clin, 2018, 68 (6): 394- 424.
doi: 10.3322/caac.21492 |
| 2 |
Ozbek E , Otunctemur A , Sahin S , et al. Renal cell carcinoma is more aggressive in Turkish patients with the metabolic syndrome[J]. Asian Pac J Cancer Prev, 2013, 14 (12): 7351- 7354.
doi: 10.7314/APJCP.2013.14.12.7351 |
| 3 | Chakiryan NH, Jiang DD, Gillis KA, et al. Real-world survival outcomes associated with first-line immunotherapy, targeted therapy, and combination therapy for metastatic clear cell renal cell carcinoma[J]. JAMA Netw Open, 2021, 4(5): e2111329[2022-03-01]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8150693/. |
| 4 |
Jonasch E , Walker CL , Rathmell WK . Clear cell renal cell carcinoma ontogeny and mechanisms of lethality[J]. Nat Rev Nephrol, 2021, 17 (4): 245- 261.
doi: 10.1038/s41581-020-00359-2 |
| 5 |
Zhang GM , Zhu Y , Ye DW . Metabolic syndrome and renal cell carcinoma[J]. World J Surg Oncol, 2014, 12, 236.
doi: 10.1186/1477-7819-12-236 |
| 6 |
Yao F , Bo Y , Zhao L , et al. Prevalence and influencing factors of metabolic syndrome among adults in China from 2015 to 2017[J]. Nutrients, 2021, 13 (12): 4475.
doi: 10.3390/nu13124475 |
| 7 |
Bovolini A , Garcia J , Andrade MA , et al. Metabolic syndrome pathophysiology and predisposing factors[J]. Int J Sports Med, 2021, 42 (3): 199- 214.
doi: 10.1055/a-1263-0898 |
| 8 |
Ewertz M , Jensen MB , Gunnarsdóttir K , et al. Effect of obesity on prognosis after early-stage breast cancer[J]. J Clin Oncol, 2011, 29 (1): 25- 31.
doi: 10.1200/JCO.2010.29.7614 |
| 9 |
Qi J , An R , Bhatti P , et al. Anti-hypertensive medications and risk of colorectal cancer: A systematic review and meta-analysis[J]. Cancer Causes Control, 2022, 33 (6): 801- 812.
doi: 10.1007/s10552-022-01570-1 |
| 10 | Yang X, Li X, Dong Y, et al. Effects of metabolic syndrome and its components on the prognosis of endometrial cancer[J]. Front Endocrinol (Lausanne), 2021, 12: 780769[2022-03-01]. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC8717682/. |
| 11 |
Bulut S , Aktas BK , Erkmen AE , et al. Metabolic syndrome prevalence in renal cell cancer patients[J]. Asian Pac J Cancer Prev, 2014, 15 (18): 7925- 7928.
doi: 10.7314/APJCP.2014.15.18.7925 |
| 12 |
Yu Y , Gong L , Ye J . The role of aberrant metabolism in cancer: Insights into the interplay between cell metabolic reprogramming, metabolic syndrome, and cancer[J]. Front Oncol, 2020, 10, 942.
doi: 10.3389/fonc.2020.00942 |
| 13 |
Luzzago S , Palumbo C , Rosiello G , et al. Metabolic syndrome predicts worse perioperative outcomes in patients treated with partial nephrectomy for renal cell carcinoma[J]. Urology, 2020, 140, 91- 97.
doi: 10.1016/j.urology.2020.02.019 |
| 14 |
Zhang Y , Wu T , Xie J , et al. Effects of metabolic syndrome on renal function after radical nephrectomy in patients with renal cell carcinoma[J]. Int Urol Nephrol, 2021, 53 (10): 2127- 2135.
doi: 10.1007/s11255-020-02759-6 |
| 15 |
Kriegmair MC , Mandel P , Porubsky S , et al. Metabolic syndrome negatively impacts the outcome of localized renal cell carcinoma[J]. Horm Cancer, 2017, 8 (2): 127- 134.
doi: 10.1007/s12672-017-0289-2 |
| 16 |
Liu Z , Wang H , Zhang L , et al. Metabolic syndrome is associated with improved cancer-specific survival in patients with localized clear cell renal cell carcinoma[J]. Transl Androl Urol, 2019, 8 (5): 507- 518.
doi: 10.21037/tau.2019.10.04 |
| 17 |
Eskelinen TJ , Kotsar A , Tammela TLJ , et al. Components of metabolic syndrome and prognosis of renal cell cancer[J]. Scand J Urol, 2017, 51 (6): 435- 441.
doi: 10.1080/21681805.2017.1352616 |
| 18 |
Du Y , Yang W , Liu H , et al. Perirenal fat as a new independent prognostic factor in patients with surgically treated clear cell renal cell carcinoma[J]. Clin Genitourin Cancer, 2022, 20 (1): e75- e80.
doi: 10.1016/j.clgc.2021.10.006 |
| 19 |
Ford E S . Prevalence of the metabolic syndrome defined by the International Diabetes Federation among adults in the U.S[J]. Diabetes Care, 2005, 28 (11): 2745- 2749.
doi: 10.2337/diacare.28.11.2745 |
| 20 |
Liu M , Wang J , Jiang B , et al. Increasing prevalence of metabolic syndrome in a Chinese elderly population: 2001-2010[J]. PLoS One, 2013, 8 (6): e66233.
doi: 10.1371/journal.pone.0066233 |
| 21 |
Capitanio U , Bensalah K , Bex A , et al. Epidemiology of renal cell carcinoma[J]. Eur Urol, 2019, 75 (1): 74- 84.
doi: 10.1016/j.eururo.2018.08.036 |
| 22 |
Miricescu D , Balan DG , Tulin A , et al. PI3K/AKT/mTOR signalling pathway involvement in renal cell carcinoma pathogenesis (Review)[J]. Exp Ther Med, 2021, 21 (5): 540.
doi: 10.3892/etm.2021.9972 |
| 23 |
Silva A , Pereira SS , Monteiro MP , et al. Effect of metabolic syndrome and individual components on colon cancer characteristics and prognosis[J]. Front Oncol, 2021, 11, 631257.
doi: 10.3389/fonc.2021.631257 |
| 24 |
Mallik R , Chowdhury TA . Metformin in cancer[J]. Diabetes Res Clin Pract, 2018, 143, 409- 419.
doi: 10.1016/j.diabres.2018.05.023 |
| 25 |
Parker AS , Lohse CM , Cheville JC , et al. Greater body mass index is associated with better pathologic features and improved outcome among patients treated surgically for clear cell renal cell carcinoma[J]. Urology, 2006, 68 (4): 741- 746.
doi: 10.1016/j.urology.2006.05.024 |
| 26 |
Wu X , Wang Q , Wang Z , et al. Association of extrarenal invasion patterns and tumor size with the differences in survival outcomes of t3a renal cell carcinoma: a proposal modified T3a stage system is needed[J]. Int J Gen Med, 2022, 15, 367- 378.
doi: 10.2147/IJGM.S344215 |
| 27 |
Dinatale RG , Xie W , Becerra MF , et al. The association between small primary tumor size and prognosis in metastatic renal cell carcinoma: Insights from two independent cohorts of patients who underwent cytoreductive nephrectomy[J]. Eur Urol Oncol, 2020, 3 (1): 47- 56.
doi: 10.1016/j.euo.2019.10.002 |
| [1] | Shengqi ZHENG,Tianchi HUA,Guicao YIN,Wei ZHANG,Ye YAO,Yifan LI. Association between the triglyceride-glucose index and the incidence of nephrolithiasis in male individuals [J]. Journal of Peking University (Health Sciences), 2024, 56(4): 610-616. |
| [2] | Junqi SU,Xiaoying WANG,Zhiqiang SUN. Establishment and verification of a prognostic nomogram for survival of tongue squamous cell carcinoma patients who underwent cervical dissection [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 120-130. |
| [3] | Yun-chong LIU,Zong-long WU,Li-yuan GE,Tan DU,Ya-qian WU,Yi-meng SONG,Cheng LIU,Lu-lin MA. Mechanism of nuclear protein 1 in the resistance to axitinib in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(5): 781-792. |
| [4] | Meng-jie CUI,Qi MA,Man-man CHEN,Tao MA,Xin-xin WANG,Jie-yu LIU,Yi ZHANG,Li CHEN,Jia-nuo JIANG,Wen YUAN,Tong-jun GUO,Yan-hui DONG,Jun MA,Yi XING. Association between different growth patterns and metabolic syndrome in children and adolescents aged 7 to 17 years [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 415-420. |
| [5] | Tian-yu CAI,Zhen-peng ZHU,Chun-ru XU,Xing JI,Tong-de LV,Zhen-ke GUO,Jian LIN. Expression and significance of fibroblast growth factor receptor 2 in clear cell renal cell carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 628-635. |
| [6] | LIU Miao, HE Yao, JIANG Bin, WU Lei, WANG Jian-Hua, YANG Shan-Shan, WANG Yi-Yan, LI Xiao-Ying. Relationship between brachial-ankle pulse wave velocity and metabolic syndrome among the elderly in a Beijing community and the gender difference [J]. Journal of Peking University(Health Sciences), 2014, 46(3): 429-434. |
| [7] | YAN Rui, LUAN Qing-Xian, LIU Li-Sheng, WANG Xing-Yu, LI Peng, SHA Yue-Qin. Association between chronic periodontitis and metabolic syndrome related mitochondria single nucleotide polymorphism [J]. Journal of Peking University(Health Sciences), 2014, 46(2): 264-268. |
|
||