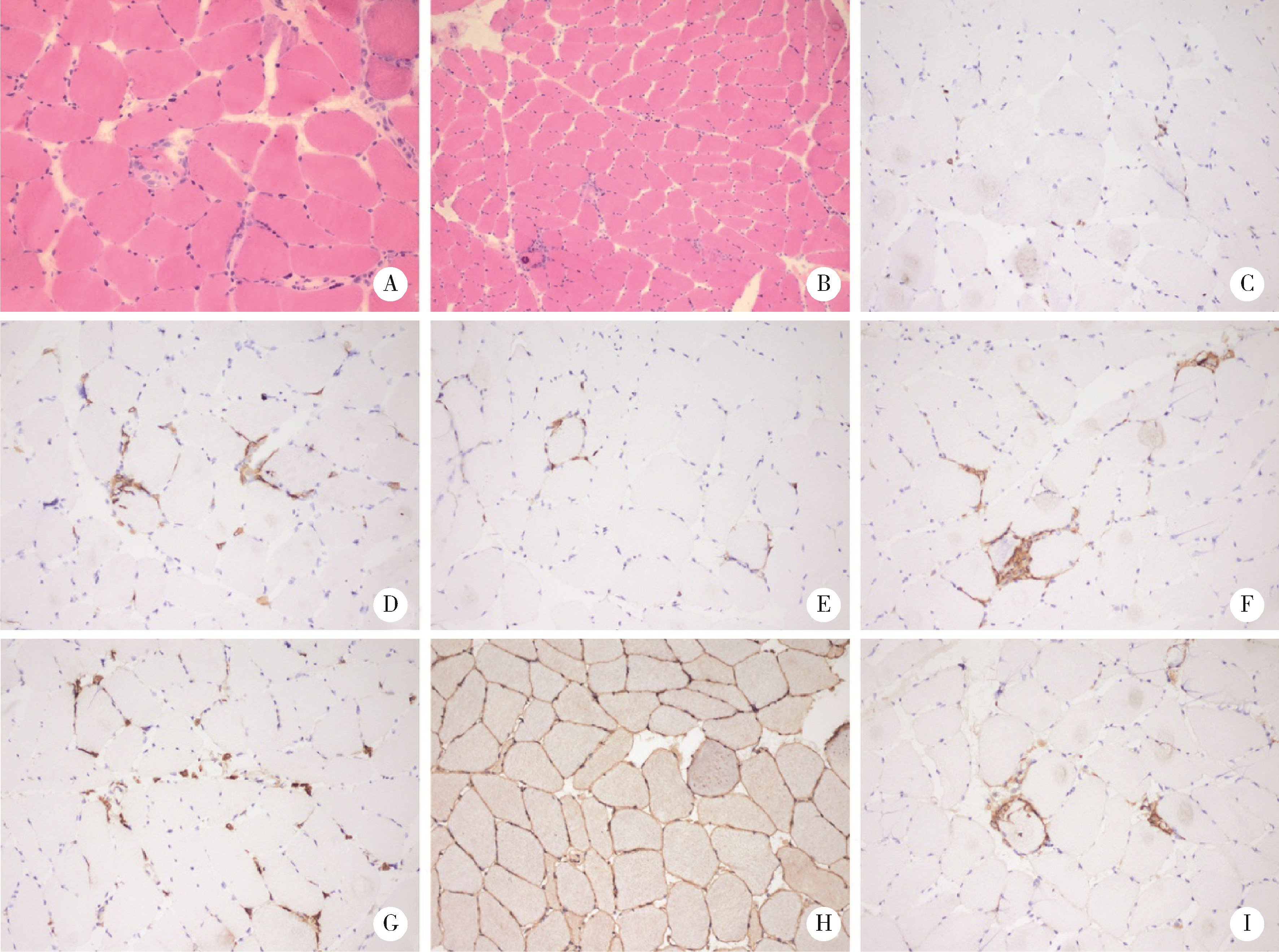
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (4): 644-651. doi: 10.19723/j.issn.1671-167X.2022.04.010
Previous Articles Next Articles
Clinical features of immune checkpoint inhibitor-related myositis in patients with urological cancer
Yi-cen YING,Qi TANG*( ),Kai-wei YANG,Yue MI,Yu FAN,Wei YU,Yi SONG,Zhi-song HE,Li-qun ZHOU,Xue-song LI*(
),Kai-wei YANG,Yue MI,Yu FAN,Wei YU,Yi SONG,Zhi-song HE,Li-qun ZHOU,Xue-song LI*( )
)
- Department of Urology, Peking University First Hospital; Institute of Urology, Peking University; National Urological Cancer Center, Beijing 100034, China
CLC Number:
- R737
| 1 |
Arnaud-Coffin P , Maillet D , Gan HK , et al. A systematic review of adverse events in randomized trials assessing immune checkpoint inhibitors[J]. Int J Cancer, 2019, 145 (3): 639- 648.
doi: 10.1002/ijc.32132 |
| 2 |
Naidoo J , Page DB , Li BT , et al. Toxicities of the anti-PD-1 and anti-PD-L1 immune checkpoint antibodies[J]. Ann Oncol, 2015, 26 (12): 2375- 2391.
doi: 10.1093/annonc/mdv383 |
| 3 | 中国临床肿瘤学会指南工作委员会. 免疫检查点抑制剂相关的毒性管理指南[M]. 2019版 北京: 人民卫生出版社, 2019: 4. |
| 4 | NCCN Guidelines Version 1. 2022 management of immunotherapy-related toxicities[EB/OL]. [2022-02-28]. https://www.nccn.org/professionals/physician_gls/pdf/immunotherapy.pdf. |
| 5 |
Vaddepally RK , Kharel P , Pandey R , et al. Review of indications of FDA-approved immune checkpoint inhibitors per NCCN guidelines with the level of evidence[J]. Cancers (Basel), 2020, 12 (3): 738.
doi: 10.3390/cancers12030738 |
| 6 |
Ernstoff MS , Gandhi S , Pandey M , et al. Challenges faced when identifying patients for combination immunotherapy[J]. Future Oncol, 2017, 13 (18): 1607- 1618.
doi: 10.2217/fon-2017-0218 |
| 7 |
Allenbach Y , Anquetil C , Manouchehri A , et al. Immune checkpoint inhibitor-induced myositis, the earliest and most lethal complication among rheumatic and musculoskeletal toxicities[J]. Autoimmun Rev, 2020, 19 (8): 102586.
doi: 10.1016/j.autrev.2020.102586 |
| 8 |
Aldrich J , Pundole X , Tummala S , et al. Inflammatory myositis in cancer patients receiving immune checkpoint inhibitors[J]. Arthritis Rheumatol, 2021, 73 (5): 866- 874.
doi: 10.1002/art.41604 |
| 9 | Moslehi JJ , Salem JE , Sosman JA , et al. Increased reporting of fatal immune checkpoint inhibitor-associated myocarditis[J]. Lancet, 2018, 391 (10124): 933. |
| 10 |
Matas-García A , Milisenda JC , Selva-O'Callaghan A , et al. Emerging PD-1 and PD-1L inhibitors-associated myopathy with a characteristic histopathological pattern[J]. Autoimmun Rev, 2020, 19 (2): 102455.
doi: 10.1016/j.autrev.2019.102455 |
| 11 |
Liewluck T , Kao JC , Mauermann ML . PD-1 Inhibitor-associated myopathies: Emerging immune-mediated myopathies[J]. J Immunother, 2018, 41 (4): 208- 211.
doi: 10.1097/CJI.0000000000000196 |
| 12 |
Johnson DB , Balko JM , Compton ML , et al. Fulminant myocarditis with combination immune checkpoint blockade[J]. N Engl J Med, 2016, 375 (18): 1749- 1755.
doi: 10.1056/NEJMoa1609214 |
| 13 |
Mahmood SS , Fradley MG , Cohen JV , et al. Myocarditis in patients treated with immune checkpoint inhibitors[J]. J Am Coll Cardiol, 2018, 71 (16): 1755- 1764.
doi: 10.1016/j.jacc.2018.02.037 |
| 14 |
Moreira A , Loquai C , Pföhler C , et al. Myositis and neuromuscular side-effects induced by immune checkpoint inhibitors[J]. Eur J Cancer, 2019, 106, 12- 23.
doi: 10.1016/j.ejca.2018.09.033 |
| 15 |
Wang DY , Salem JE , Cohen JV , et al. Fatal toxic effects associated with immune checkpoint inhibitors: A systematic review and meta-analysis[J]. JAMA Oncol, 2018, 4 (12): 1721- 1728.
doi: 10.1001/jamaoncol.2018.3923 |
| 16 |
Awadalla M , Mahmood SS , Groarke JD , et al. Global longitudinal strain and cardiac events in patients with immune checkpoint inhibitor-related myocarditis[J]. J Am Coll Cardiol, 2020, 75 (5): 467- 478.
doi: 10.1016/j.jacc.2019.11.049 |
| 17 |
Dolladille C , Ederhy S , Allouche S , et al. Late cardiac adverse events in patients with cancer treated with immune checkpoint inhibitors[J]. J Immunother Cancer, 2020, 8 (1): e000261.
doi: 10.1136/jitc-2019-000261 |
| 18 |
Salem JE , Manouchehri A , Moey M , et al. Cardiovascular toxicities associated with immune checkpoint inhibitors: an observational, retrospective, pharmacovigilance study[J]. Lancet Oncol, 2018, 19 (12): 1579- 1589.
doi: 10.1016/S1470-2045(18)30608-9 |
| 19 |
Weill A , Delyon J , Descamps V , et al. Treatment strategies and safety of rechallenge in the setting of immune checkpoint inhibitors-related myositis: a national multicentre study[J]. Rheumatology (Oxford), 2021, 60 (12): 5753- 5764.
doi: 10.1093/rheumatology/keab249 |
| 20 |
Pollack MH , Betof A , Dearden H , et al. Safety of resuming anti-PD-1 in patients with immune-related adverse events (irAEs) during combined anti-CTLA-4 and anti-PD1 in metastatic melanoma[J]. Ann Oncol, 2018, 29 (1): 250- 255.
doi: 10.1093/annonc/mdx642 |
| [1] | Xiao-yan XING,Jun-xiao ZHANG,Feng-yun-zhi ZHU,Yi-fan WANG,Xin-yao ZHOU,Yu-hui LI. Clinical analysis of 5 cases of dermatomyositis complicated with macrophage activation syndrome [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1214-1218. |
| [2] | Sheng-jie LIU,Hui-min HOU,Zheng-tong LV,Xin DING,Lu WANG,Lei ZHANG,Ming LIU. Bipolar androgen therapy followed by immune checkpoint inhibitors in metastatic castration resistant prostate cancer: A report of 4 cases [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 766-769. |
| [3] | Cai-peng QIN,Yu-xuan SONG,Meng-ting DING,Fei WANG,Jia-xing LIN,Wen-bo YANG,Yi-qing DU,Qing LI,Shi-jun LIU,Tao XU. Establishment of a mutation prediction model for evaluating the efficacy of immunotherapy in renal carcinoma [J]. Journal of Peking University (Health Sciences), 2022, 54(4): 663-668. |
| [4] | GU Yang-chun,LIU Ying,XIE Chao,CAO Bao-shan. Pituitary immune-related adverse events induced by programmed cell death protein 1 inhibitors in advanced lung cancer patients: A report of 3 cases [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 369-375. |
| [5] | YI Wen-xia,WEI Cui-jie,WU Ye,BAO Xin-hua,XIONG Hui,CHANG Xing-zhi. Long-term rituximab treatment of refractory idiopathic inflammatory myopathy: A report of 3 cases [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1191-1195. |
| [6] | XIAO Yun-shu,ZHU Feng-yun-zhi,LUO Lan,XING Xiao-yan,LI Yu-hui,ZHANG Xue-wu,SHEN Dan-hua. Clinical and immunological characteristics of 88 cases of overlap myositis [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1088-1093. |
| [7] | LUO Lan,XING Xiao-yan,XIAO Yun-shu,CHEN Ke-yan,ZHU Feng-yun-zhi,ZHANG Xue-wu,LI Yu-hui. Clinical and immunological characteristics of patients with anti-synthetase syndrome complicated with cardiac involvement [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1078-1082. |
| [8] | ZHANG Pu-li,YANG Hong-xia,ZHANG Li-ning,GE Yong-peng,PENG Qing-lin,WANG Guo-chun,LU Xin. Value of serum YKL-40 in the diagnosis of anti-MDA5-positive patients with dermatomyositis complicated with severe pulmonary injury [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1055-1060. |
| [9] | Feng-yun-zhi ZHU,Xiao-yan XING,Xiao-fei TANG,Yi-min LI,Miao SHAO,Xue-Wu ZHANG,Yu-hui LI,Xiao-lin SUN,Jing HE. Clinical and immunological characteristics of myositis complicated with thromboembolism [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 995-1000. |
| [10] | Yi-ming ZHENG,Hong-jun HAO,Yi-lin LIU,Jing GUO,Ya-wen ZHAO,Wei ZHANG,Yun YUAN. Correlation study on anti-Ro52 antibodies frequently co-occur with other myositis-specific and myositis-associated autoantibodies [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 1088-1092. |
| [11] | Yu-zhou GAN,Yu-hui LI,Li-hua ZHANG,Lin MA,Wen-wen HE,Yue-bo JIN,Yuan AN,Zhan-guo LI,Hua YE. Comparison of clinical and immunological features between clinically amyopathic dermatomyositis and typical dermatomyositis [J]. Journal of Peking University (Health Sciences), 2020, 52(6): 1001-1008. |
| [12] | Jing XU,Jing XU,He LI,Jie TANG,Jian-long SHU,Jing ZHANG,Lian-jie SHI,Sheng-guang LI. Dermatomyositis combined with IgA vasculitis: A case report [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 1173-1177. |
| [13] | Hong-xia YANG,Xiao-lan TIAN,Wei JIANG,Wen-li LI,Qing-yan LIU,Qing-lin PENG,Guo-chun WANG,Xin LU. Clinical and pathological characteristics of immune mediated necrotizing myopathy [J]. Journal of Peking University(Health Sciences), 2019, 51(6): 989-995. |
| [14] | Yi-ying YANG,Xiao-xia ZUO,Hong-lin ZHU,Si-jia LIU. Advances in epigenetic markers of dermatomyositis/polymyositis [J]. Journal of Peking University(Health Sciences), 2019, 51(2): 374-377. |
| [15] | YU Jian-feng, JIN Yue-bo, HE Jing, AN Yuan, LI Zhan-guo. Changes of serum Krebs von den Lungen-6 levels in interstitial lung disease associated with dermatomyositis and secondary Sjögren’s syndrome: a case report [J]. Journal of Peking University(Health Sciences), 2017, 49(5): 910-914. |
|
||