Journal of Peking University (Health Sciences) ›› 2021, Vol. 53 ›› Issue (2): 348-354. doi: 10.19723/j.issn.1671-167X.2021.02.020
Previous Articles Next Articles
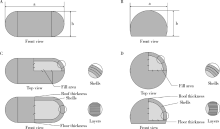
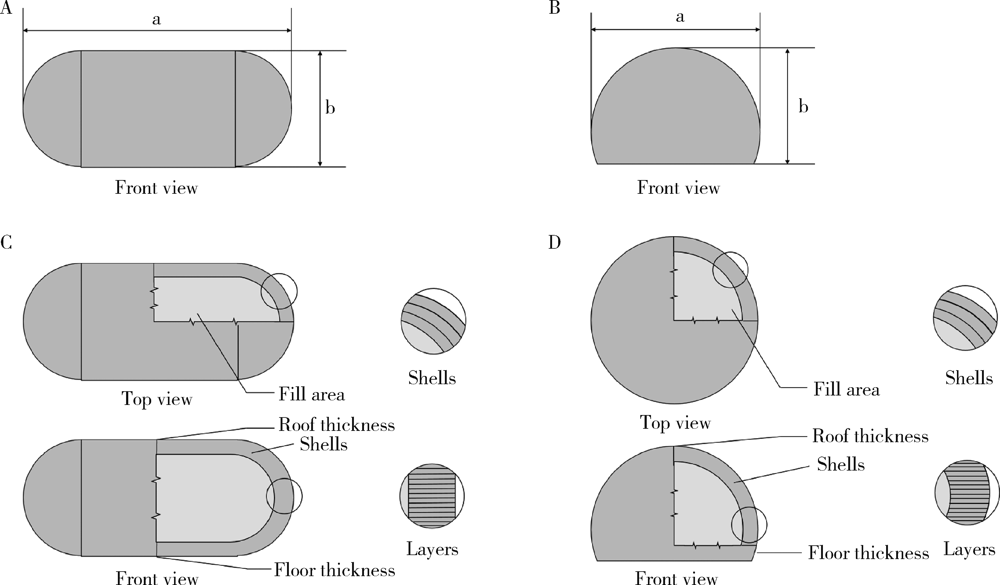
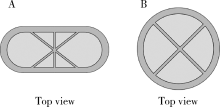
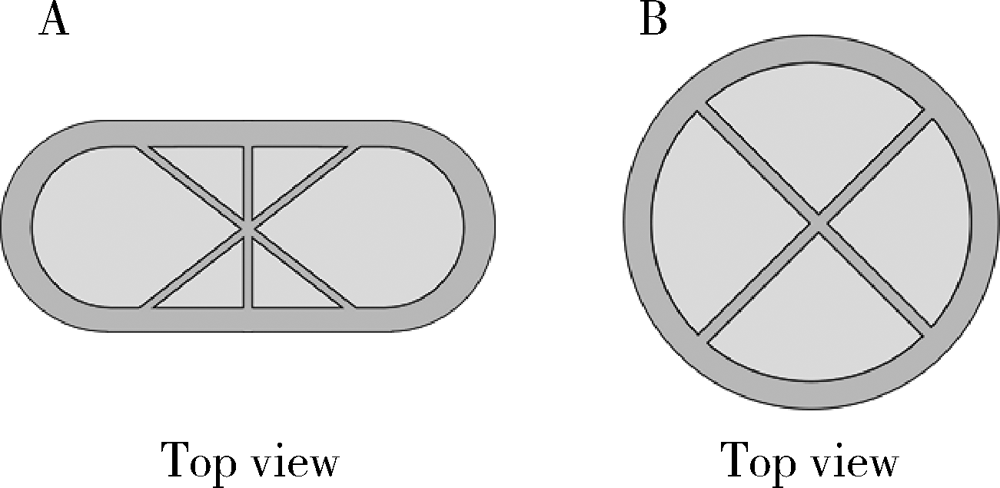
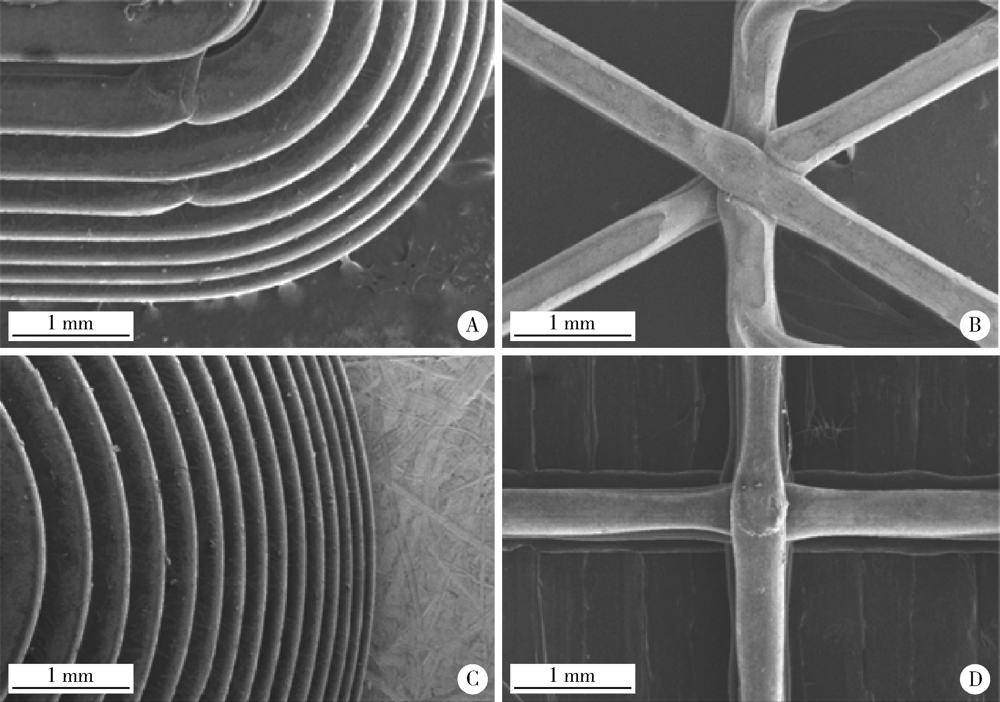
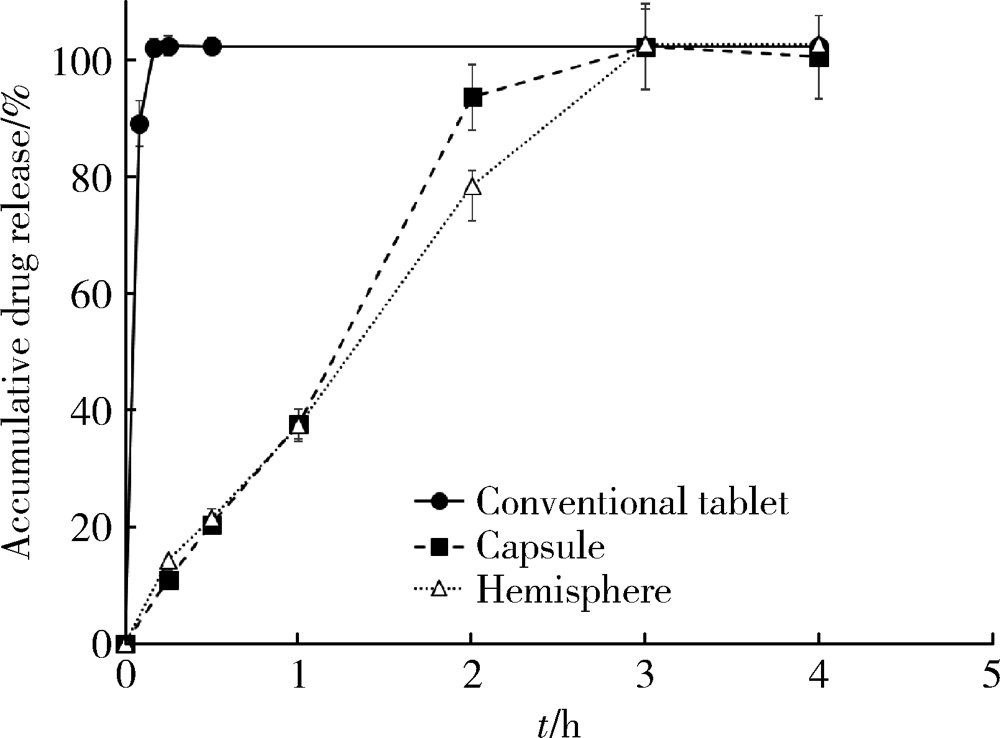
Preparation and in vitro evaluation of fused deposition modeling 3D printed verapa-mil hydrochloride gastric floating formulations
CHEN Di1,XU Xiang-yu2,WANG Ming-rui1,LI Rui1,ZANG Gen-ao2,ZHANG Yue1,QIAN Hao-nan1,YAN Guang-rong2,Δ( ),FAN Tian-yuan1,Δ(
),FAN Tian-yuan1,Δ( )
)
- 1. Department of Pharmaceutics, Beijing Key Laboratory of Molecular Pharmaceutics and New Drug Delivery Systems, Peking University School of Pharmaceutical Sciences, Beijing 100191, China
2. School of Mechanical Engineering and Automation, Beihang University, Beijing 100191, China
CLC Number:
- R944.9
| [1] | Kaushik AY, Tiwari AK, Gaur A. Role of excipients and polyme-ric advancements in preparation of floating drug delivery systems[J]. Int J Pharm Investig, 2015,5(1):1-12. |
| [2] |
Pawar VK, Kansal S, Garg G, et al. Gastroretentive dosage forms: A review with special emphasis on floating drug delivery systems[J]. Drug Deliv, 2011,18(2):97-110.
pmid: 20958237 |
| [3] | Kotreka UK, Adeyeye MC. Gastroretentive floating drug-delivery systems: A critical review[J]. Crit Rev Ther Drug Carrier Syst, 2011,28(1):47-99. |
| [4] | Sauzet C, Claeys-Bruno M, Nicolas M, et al. An innovative floating gastro retentive dosage system: formulation and in vitro evaluation[J]. Int J Pharm, 2009,378(1/2):23-29. |
| [5] | Sungthongjeen S, Paeratakul O, Limmatvapirat S, et al. Preparation and in vitro evaluation of a multiple-unit floating drug delivery system based on gas formation technique[J]. Int J Pharm, 2006,324(2):136-143. |
| [6] |
Alexander S, Juergen S, Roland B. Gastroretentive drug delivery systems[J]. Expert Opinion on Drug Delivery, 2006,3(2):217-233.
pmid: 16506949 |
| [7] | Konta AA, Garcia-Pina M, Serrano DR. Personalised 3D printed medicines: Which techniques and polymers are more successful?[J]. Bioengineering (Basel), 2017,4(4):79-96. |
| [8] | Long J, Gholizadeh H, Lu J, et al. Application of fused deposition modelling (FDM) method of 3D printing in drug delivery[J]. Curr Pharm Des, 2017,23(3):433-439. |
| [9] | Palo M, Hollander J, Suominen J, et al. 3D printed drug delivery devices: Perspectives and technical challenges[J]. Expert Rev Med Devices, 2017,14(9):685-696. |
| [10] |
Alhnan MA, Okwuosa TC, Sadia M, et al. Emergence of 3D printed dosage forms: Opportunities and challenges[J]. Pharm Res, 2016,33(8):1817-1832.
pmid: 27194002 |
| [11] | Sawicki W, Glod J. Preparation of floating pellets with verapamil hydrochloride[J]. Acta Pol Pharm, 2004,61(3):185-190. |
| [12] | Patel A, Modasiya M, Shah D, et al. Development and in vivo floating behavior of verapamil HCl intragastric floating tablets[J]. AAPS PharmSciTech, 2009,10(1):310-315. |
| [13] | 范田园, 张悦. 一种3D打印胃漂浮制剂及其制备方法: 中国,CN106692091A[P]. 2017-05-24. |
| [14] | Srikanth MV, Rao NS, Sunil SA, et al. Statistical design and evaluation of a propranolol HCl gastric floating tablet[J]. Acta Pharmaceutica Sinica B, 2012,2(1):60-69. |
| [15] | Fu J, Yin H, Yu X, et al. Combination of 3D printing technologies and compressed tablets for preparation of riboflavin floating tablet-in-device (TiD) systems[J]. Int J Pharm, 2018,549(1/2):370-379. |
| [16] |
Goyanes A, Buanz AB, Hatton GB, et al. 3D printing of modified-release aminosalicylate (4-ASA and 5-ASA) tablets[J]. Eur J Pharm Biopharm, 2015,89:157-162.
pmid: 25497178 |
| [17] |
Fuenmayor E, Forde M, Healy AV, et al. Comparison of fused-filament fabrication to direct compression and injection molding in the manufacture of oral tablets[J]. Int J Pharm, 2019,558:328-340.
pmid: 30659922 |
| [18] | Yang Y, Wang H, Li H, et al. 3D printed tablets with internal scaffold structure using ethyl cellulose to achieve sustained ibuprofen release[J]. Eur J Pharm Sci, 2018,115:11-18. |
| [19] | Tagami T, Nagata N, Hayashi N, et al. Defined drug release from 3D-printed composite tablets consisting of drug-loaded polyvinylalcohol and a water-soluble or water-insoluble polymer filler[J]. Int J Pharm, 2018,543(1/2):361-367. |
| [20] | Solanki NG, Tahsin M, Shah AV, et al. Formulation of 3D printed tablet for rapid drug release by fused deposition modeling: Screening polymers for drug release, drug-polymer miscibility and printability[J]. J Pharm Sci, 2018,107(1):390-401. |
| [21] |
Arafat B, Wojsz M, Isreb A, et al. Tablet fragmentation without a disintegrant: A novel design approach for accelerating disintegration and drug release from 3D printed cellulosic tablets[J]. Eur J Pharm Sci, 2018,118:191-199.
pmid: 29559404 |
| [22] |
Kadry H, Al-Hilal TA, Keshavarz A, et al. Multi-purposable filaments of HPMC for 3D printing of medications with tailored drug release and timed-absorption[J]. Int J Pharm, 2018,544(1):285-296.
doi: 10.1016/j.ijpharm.2018.04.010 pmid: 29680281 |
| [23] |
Sadia M, Arafat B, Ahmed W, et al. Channelled tablets: An innovative approach to accelerating drug release from 3D printed tablets[J]. J Control Release, 2018,269:355-363.
pmid: 29146240 |
| [24] | Tagami T, Fukushige K, Ogawa E, et al. 3D Printing factors important for the fabrication of polyvinylalcohol filament-based tablets[J]. Biol Pharm Bull, 2017,40(3):357-364. |
| [25] |
Goyanes A, Robles Martinez P, Buanz A, et al. Effect of geometry on drug release from 3D printed tablets[J]. Int J Pharm, 2015,494(2):657-663.
pmid: 25934428 |
| [26] |
Zhang J, Yang W, Vo AQ, et al. Hydroxypropyl methylcellulose-based controlled release dosage by melt extrusion and 3D printing: Structure and drug release correlation[J]. Carbohydr Polym, 2017,177:49-57.
pmid: 28962795 |
| [27] |
Goyanes A, Wang J, Buanz A, et al. 3D printing of medicines: Engineering novel oral devices with unique design and drug delease characteristics[J]. Mol Pharm, 2015,12(11):4077-4084.
pmid: 26473653 |
| [28] |
Lunio R, Sawicki W. Influence of the components of Kollicoat SR film on mechanical properties of floating pellets from the point of view of tableting[J]. Pharmazie, 2008,63(10):731-735.
pmid: 18972835 |
| [29] |
Yoshida MI, Gomes EC, Soares CD, et al. Thermal analysis applied to verapamil hydrochloride characterization in pharmaceutical formulations[J]. Molecules, 2010,15(4):2439-2452.
pmid: 20428054 |
| [30] |
Soppimath KS, Kulkarni AR, Aminabhavi TM. Development of hollow microspheres as floating controlled-release systems for car-diovascular drugs: Preparation and release characteristics[J]. Drug Dev Ind Pharm, 2001,27(6):507-515.
pmid: 11548857 |
| [31] |
Durig T, Fassihi R. Evaluation of floating and sticking extended release delivery systems: An unconventional dissolution test[J]. J Control Release, 2000,67(1):37-44.
pmid: 10773327 |
| [32] |
Sawicki W, Lunio R, Walentynowicz O, et al. Influence of the type of cellulose on properties of multi-unit target releasing in sto-mach dosage form with verapamil hydrochloride[J]. Acta Pol Pharm, 2007,64(1):81-88.
pmid: 17665855 |
| [1] | Xinxin ZHAN,Lulu CAO,Dong XIANG,Hao TANG,Dandan XIA,Hong LIN. Effect of printing orientation on physical and mechanical properties of 3D printing prosthodontic base resin materials [J]. Journal of Peking University (Health Sciences), 2024, 56(2): 345-351. |
| [2] | Liang LYU,Mingjin ZHANG,Aonan WEN,Yijiao ZHAO,Yong WANG,Jing LI,Gengchen YANG,Dawei LIU. Preliminary evaluation of chin symmetry with three dimentional soft tissue spatial angle wireframe template [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 106-110. |
| [3] | Bochun MAO,Yajing TIAN,Xuedong WANG,Jing LI,Yanheng ZHOU. Soft and hard tissue changes of hyperdivergent class Ⅱ patients before and after orthodontic extraction treatment [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 111-119. |
| [4] | Xiaotong LING,Liuyang QU,Danni ZHENG,Jing YANG,Xuebing YAN,Denggao LIU,Yan GAO. Three-dimensional radiographic features of calcifying odontogenic cyst and calcifying epithelial odontogenic tumor [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 131-137. |
| [5] | Panpan HU,Yan LI,Xiao LIU,Yanchao TANG,Zihe LI,Zhongjun LIU. Clinical outcomes of 3D-printing stand-alone artificial vertebral body in anterior cervical surgeries [J]. Journal of Peking University (Health Sciences), 2024, 56(1): 161-166. |
| [6] | Wen ZHANG,Xiao-jing LIU,Zi-li LI,Yi ZHANG. Effect of alar base cinch suture based on anatomic landmarks on the morphology of nasolabial region in patients after orthognathic surgery [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 736-742. |
| [7] | Meng-en OU,Yun DING,Wei-feng TANG,Yong-sheng ZHOU. Three-dimensional finite element analysis of cement flow in abutment margin-crown platform switching [J]. Journal of Peking University (Health Sciences), 2023, 55(3): 548-552. |
| [8] | Ao-nan WEN,Wei LIU,Da-wei LIU,Yu-jia ZHU,Ning XIAO,Yong WANG,Yi-jiao ZHAO. Preliminary evaluation of the trueness of 5 chairside 3D facial scanning techniques [J]. Journal of Peking University (Health Sciences), 2023, 55(2): 343-350. |
| [9] | Shi-kai XIONG,Wei-li SHI,An-hong WANG,Xing XIE,Qin-wei GUO. Radiographic diagnosis of distal fibula avulsion fractures: Comparison of ankle X-ray and three-dimensional reconstruction of CT [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 156-159. |
| [10] | Zi-xiang GAO,Yong WANG,Ao-nan WEN,Yu-jia ZHU,Qing-zhao QIN,Yun ZHANG,Jing WANG,Yi-jiao ZHAO. Automatic determination of mandibular landmarks based on three-dimensional mandibular average model [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 174-180. |
| [11] | ABUDUREHEMAN Kaidierya,Rong-geng ZHANG,Hao-nan QIAN,Zhen-yang ZOU,YESITAO Danniya,Tian-yuan FAN. Preparation and in vitro evaluation of FDM 3D printed theophylline tablets with personalized dosage [J]. Journal of Peking University (Health Sciences), 2022, 54(6): 1202-1207. |
| [12] | Hai-ying XING,Yu-hui CHEN,Ke XU,Dian-dian HUANG,Qing PENG,Ran LIU,Wei SUN,Yi-ning HUANG. Evaluation of carotid atherosclerotic plaques by vascular plaque quantification (VPQ) technology of three-dimensional ultrasonography [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 991-999. |
| [13] | Zhi-sheng LI,Hao-nan QIAN,Tian-yuan FAN. Preparation and in vitro evaluation of fused deposition modeling 3D printed compound tablets of captopril and hydrochlorothiazide [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 572-577. |
| [14] | LIU Si-min,ZHAO Yi-jiao,WANG Xiao-yan,WANG Zu-hua. In vitro evaluation of positioning accuracy of trephine bur at different depths by dynamic navigation [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 146-152. |
| [15] | QIU Shu-ting,ZHU Yu-jia,WANG Shi-min,WANG Fei-long,YE Hong-qiang,ZHAO Yi-jiao,LIU Yun-song,WANG Yong,ZHOU Yong-sheng. Preliminary clinical application verification of complete digital workflow of design lips symmetry reference plane based on posed smile [J]. Journal of Peking University (Health Sciences), 2022, 54(1): 193-199. |
|
||