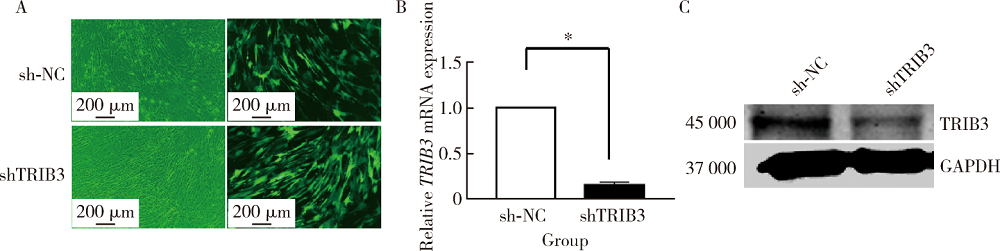
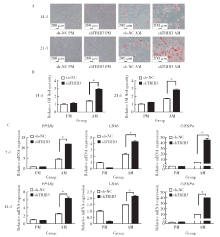
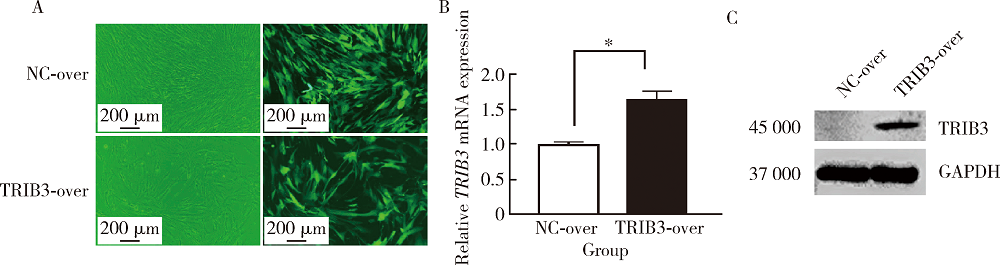
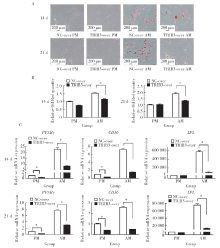
Journal of Peking University(Health Sciences) ›› 2020, Vol. 52 ›› Issue (1): 1-9. doi: 10.19723/j.issn.1671-167X.2020.01.001
Tribbles pseudokinase 3 inhibits the adipogenic differentiation of human adipose-derived mesenchymal stem cells
Xiang-song BAI,Long-wei LV( ),Yong-sheng ZHOU
),Yong-sheng ZHOU
- Department of Prosthodontics, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
CLC Number:
- R329.2
| [1] | Rubin JP, Marra KG . Adipose stem cell therapy for soft tissue reconstruction[J]. Lancet, 2013,382(9898):1077-1079. |
| [2] | Patrick CJ, Chauvin PB, Hobley J , et al. Preadipocyte seeded PLGA scaffolds for adipose tissue engineering[J]. Tissue Eng, 1999,5(2):139-151. |
| [3] | Paschos NK, Brown WE, Eswaramoorthy R , et al. Advances in tissue engineering through stem cell-based co-culture[J]. J Tissue Eng Regen Med, 2015,9(5):488-503. |
| [4] | Cossu G, Birchall M, Brown T , et al. Lancet Commission: Stem cells and regenerative medicine[J]. Lancet, 2018,391(10123):883-910. |
| [5] | Zuk PA, Zhu M, Mizuno H , et al. Multilineage cells from human adipose tissue: Implications for cell-based therapies[J]. Tissue Eng, 2001,7(2):211-228. |
| [6] | Zuk PA, Zhu M, Ashjian P , et al. Human adipose tissue is a source of multipotent stem cells[J]. Mol Biol Cell, 2002,13(12):4279-4295. |
| [7] | Gentile P, Orlandi A, Scioli MG , et al. Concise review: Adipose-derived stromal vascular fraction cells and platelet-rich plasma: Basic and clinical implications for tissue engineering therapies in regenerative surgery[J]. Stem Cells Transl Med, 2012,1(3):230-236. |
| [8] | Lv LW, Liu YS, Zhang P , et al. Transcriptomics and functional analysis of graphene-guided osteogenic differentiation of mesenchymal stem cells[J]. Chin J Dent Res, 2018,21(2):101-111. |
| [9] | Eyers PA, Keeshan K, Kannan N . Tribbles in the 21st Century: The evolving roles of tribbles pseudokinases in biology and disease[J]. Trends Cell Biol, 2017,27(4):284-298. |
| [10] | Ord T, Ord T . Mammalian pseudokinase TRIB3 in normal physi-ology and disease: Charting the progress in old and new avenues[J]. Curr Protein Pept Sci, 2017,18(8):819-842. |
| [11] | Prudente S, Sesti G, Pandolfi A , et al. The mammalian tribbles homolog TRIB3, glucose homeostasis, and cardiovascular diseases[J]. Endocr Rev, 2012,33(4):526-546. |
| [12] | Liu G, Jin S, Hu Y , et al. Disease status affects the association between rs4813620 and the expression of Alzheimer’s disease susceptibility gene TRIB3[J]. Proc Natl Acad Sci USA, 2018,115(45):E10519-E10520. |
| [13] | Hua F, Li K, Yu JJ , et al. The TRIB3-SQSTM1 interaction mediates metabolic stress-promoted tumorigenesis and progression via suppressing autophagic and proteasomal degradation[J]. Auto-phagy, 2015,11(10):1929-1931. |
| [14] | Mondal D, Mathur A, Chandra PK . Tripping on TRIB3 at the junction of health, metabolic dysfunction and cancer[J]. Biochimie, 2016,124:34-52. |
| [15] | Kusminski CM, Bickel PE, Scherer PE . Targeting adipose tissue in the treatment of obesity-associated diabetes[J]. Nat Rev Drug Discov, 2016,15(9):639-660. |
| [16] | Picon-Ruiz M, Morata-Tarifa C, Valle-Goffin JJ , et al. Obesity and adverse breast cancer risk and outcome: Mechanistic insights and strategies for intervention[J]. CA Cancer J Clin, 2017,67(5):378-397. |
| [17] | Bezy O, Vernochet C, Gesta S , et al. TRB3 blocks adipocyte differentiation through the inhibition of C/EBPbeta transcriptional activity[J]. Mol Cell Biol, 2007,27(19):6818-6831. |
| [18] | Wang X, Gao L, Han Y , et al. Silicon-enhanced adipogenesis and angiogenesis for vascularized adipose tissue engineering[J]. Adv Sci (Weinh), 2018,5(11):1800776. |
| [19] | Levi B, Longaker MT . Concise review: Adipose-derived stromal cells for skeletal regenerative medicine[J]. Stem Cells, 2011,29(4):576-582. |
| [20] | Zhu Y, Zhang P, Gu RL , et al. Origin and clinical applications of neural crest-derived dental stem cells[J]. Chin J Dent Res, 2018,21(2):89-100. |
| [21] | Lu Z, Yuan Y, Gao J , et al. Adipose tissue extract promotes adipose tissue regeneration in an adipose tissue engineering chamber model[J]. Cell Tissue Res, 2016,364(2):289-298. |
| [22] | Volz AC, Huber B, Kluger PJ . Adipose-derived stem cell differentiation as a basic tool for vascularized adipose tissue engineering[J]. Differentiation, 2016,92(1/2):52-64. |
| [23] | Gomillion CT, Burg KJ . Stem cells and adipose tissue engineering[J]. Biomaterials, 2006,27(36):6052-6063. |
| [24] | Scott MA, Nguyen VT, Levi B , et al. Current methods of adipogenic differentiation of mesenchymal stem cells[J]. Stem Cells Dev, 2011,20(10):1793-1804. |
| [25] | Saltiel AR . Putting the brakes on insulin signaling[J]. N Engl J Med, 2003,349(26):2560-2562. |
| [26] | Qi L, Heredia JE, Altarejos JY , et al. TRB3 links the E3 ubi-quitin ligase COP1 to lipid metabolism[J]. Science, 2006,312(5781):1763-1766. |
| [27] | Brazil DP, Hemmings BA . Ten years of protein kinase B signalling: A hard Akt to follow[J]. Trends Biochem Sci, 2001,26(11):657-664. |
| [28] | Cho H, Mu J, Kim JK , et al. Insulin resistance and a diabetes mellitus-like syndrome in mice lacking the protein kinase Akt2 (PKB beta)[J]. Science, 2001,292(5522):1728-1731. |
| [29] | Du K, Herzig S, Kulkarni RN , et al. TRB3: A tribbles homolog that inhibits Akt/PKB activation by insulin in liver[J]. Science, 2003,300(5625):1574-1577. |
| [30] | Park JS, Kim M, Song NJ , et al. A reciprocal role of the Smad4-Taz axis in osteogenesis and adipogenesis of mesenchymal stem cells[J]. Stem Cells, 2019,37(3):368-381. |
| [31] | Hyvari L, Ojansivu M, Juntunen M , et al. Focal adhesion kinase and ROCK signaling are switch-like regulators of human adipose stem cell differentiation towards osteogenic and adipogenic lineages[J]. Stem Cells Int, 2018, 9(2018-09-12)[2018-12-12]. . |
| [32] | Liu X, He J, Zhang S , et al. Adipose stem cells controlled by surface chemistry[J]. J Tissue Eng Regen Med, 2013,7(2):112-117. |
| [33] | Furuhata Y, Yoshitomi T, Kikuchi Y , et al. Osteogenic lineage commitment of adipose-derived stem cells is predetermined by three-dimensional cell accumulation on micropatterned surface[J]. ACS Appl Mater Interfaces, 2017,9(11):9339-9347. |
| [34] | Takahashi Y, Ohoka N, Hayashi H , et al. TRB3 suppresses adipocyte differentiation by negatively regulating PPARgamma transcriptional activity[J]. J Lipid Res, 2008,49(4):880-892. |
| [35] | Chan MC, Hilyard AC, Wu C , et al. Molecular basis for antagonism between PDGF and the TGFbeta family of signalling pathways by control of miR-24 expression[J]. EMBO J, 2010,29(3):559-573. |
| [36] | Roy L, Bikorimana E, Lapid D , et al. MiR-24 is required for hematopoietic differentiation of mouse embryonic stem cells[J]. PLoS Genet, 2015,11(1):e1004959. |
| [37] | Kim S, Hata A, Kang H . Down-regulation of miR-96 by bone morphogenetic protein signaling is critical for vascular smooth muscle cell phenotype modulation[J]. J Cell Biochem, 2014,115(5):889-895. |
| [38] | Eom HJ, Chatterjee N, Lee J , et al. Integrated mRNA and micro RNA profiling reveals epigenetic mechanism of differential sensiti-vity of Jurkat T cells to AgNPs and Ag ions[J]. Toxicol Lett, 2014,229(1):311-318. |
| [39] | Liu X, Zhao J, Liu Q , et al. MicroRNA-124 promotes hepatic triglyceride accumulation through targeting tribbles homolog 3[J]. Sci Rep, 2016,6:37170. |
| [1] | SHUAI Ting,LIU Juan,GUO Yan-yan,JIN Chan-yuan. Knockdown of long non-coding RNA MIR4697 host gene inhibits adipogenic differentiation in bone marrow mesenchymal stem cells [J]. Journal of Peking University (Health Sciences), 2022, 54(2): 320-326. |
|
||