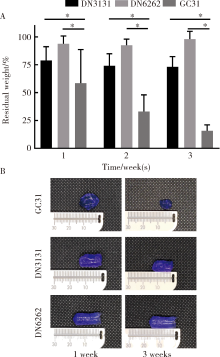
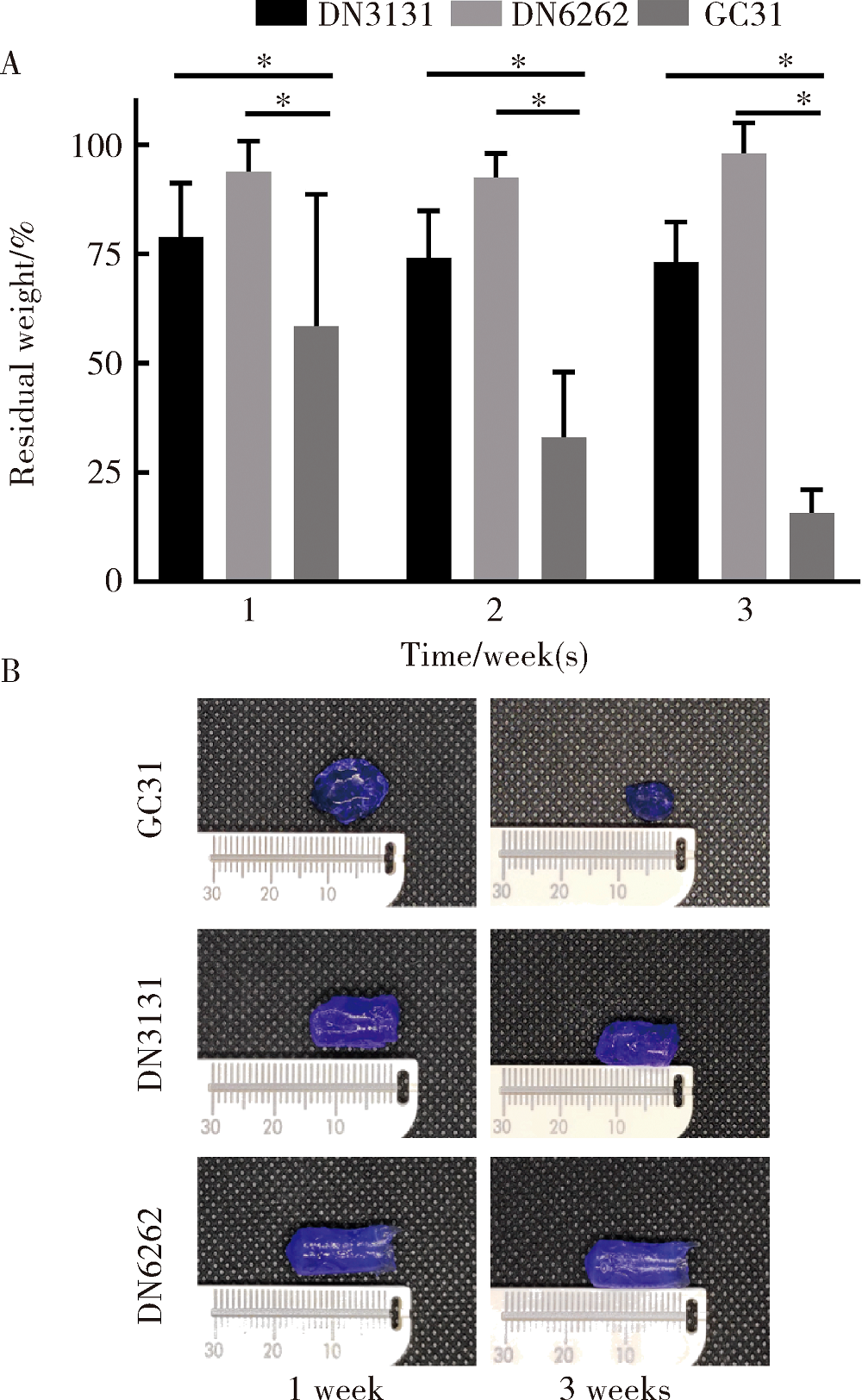
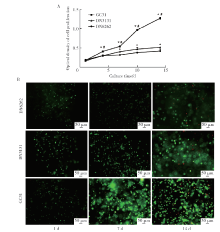
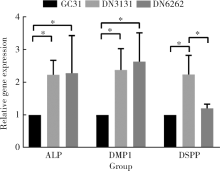
Journal of Peking University(Health Sciences) ›› 2020, Vol. 52 ›› Issue (1): 10-17. doi: 10.19723/j.issn.1671-167X.2020.01.002
Previous Articles Next Articles
Effects of the injectable glycol-chitosan based hydrogel on the proliferation and differentiation of human dental pulp cells
Chun-ling CAO1,Cong-chong YANG1,Xiao-zhong QU2,Bing HAN1,△( ),Xiao-yan WANG1,△(
),Xiao-yan WANG1,△( )
)
- 1. Department of Cariology and Endodontology, Peking University School and Hospital of Stomatology & National Clinical Research Center for Oral Diseases & National Engineering Laboratory for Digital and Material Technology of Stomatology & Beijing Key Laboratory of Digital Stomatology, Beijing 100081, China
2. College of Materials Science and Opto-electronic Technology, University of Chinese Academy of Sciences, Beijing 100049, China
CLC Number:
- R783.1
| [1] | 孙艳艳, 袁梦桐, 胡伟平 . 牙髓干细胞的研究与应用[J]. 实用口腔医学杂志, 2016,32(3):426-429. |
| [2] | Xuan K, Li B, Guo H , et al. Deciduous autologous tooth stem cells regenerate dental pulp after implantation into injured teeth[J]. Sci Trans Med, 2018,10(455):3227. |
| [3] | Galler KM, Hartgerink JD, Cavender A C , et al. A customized self-assembling peptide hydrogel for dental pulp tissue engineering[J]. Tissue Eng Part A, 2012,18(1/2):176-184. |
| [4] | Qu T, Jing J, Ren Y , et al. Complete pulpodentin complex re-generation by modulating the stiffness of biomimetic matrix[J]. Acta Biomater, 2015,16(1):60-70. |
| [5] | Smith JG, Smith AJ, Shelton RM , et al. Dental pulp cell behavior in biomimetic environments[J]. J Dent Res, 2015,94(11):1552-1559. |
| [6] | Gillette BM, Jensen JA, Wang M , et al. Dynamic hydrogels: switching of 3D microenvironments using two-component naturally derived extracellular matrices[J]. Adv Mater, 2010,22(6):686-691. |
| [7] | Xu X, Gu Z, Chen X , et al. An injectable and thermosensitive hydrogel: Promoting periodontal regeneration by controlled-release of aspirin and erythropoietin[J]. Acta Biomater, 2019,86:235-246. |
| [8] | Yan Y, Li M, Yang D , et al. Construction of injectable double-network hydrogels for cell delivery[J]. Biomacromolecules, 2017,18(7):2128-2138. |
| [9] | Lee KY, Mooney DJ . Hydrogels for tissue engineering[J]. Chem Rev, 2001,101(7):1869-1880. |
| [10] | Jones TD, Kefi A, Sun S, et al. An optimized injectable hydrogel scaffold supports human dental pulp stem cell viability and spreading[J/OL]. Adv Med, 2016, 2016: 7363579(2016-05-16)[2019-09-01]. . |
| [11] | Her GJ, Wu H, Chen M , et al. Control of three-dimensional substrate stiffness to manipulate mesenchymal stem cell fate toward neuronal or glial lineages[J]. Acta Biomater, 2013,9(2):5170-5180. |
| [12] | Slaughter BV, Khurshid SS, Fisher OZ , et al. Hydrogels in regenerative medicine[J]. Adv Mater, 2009,21(32/33):3307-3329. |
| [13] | Dash M, Chiellini F, Ottenbrite RM , et al. Chitosan: a versatile semi-synthetic polymer in biomedical applications[J]. Prog Polym Sci, 2011,36(8):981-1014. |
| [14] | Zou H, Wang G, Song F , et al. Investigation of human dental pulp cells on a potential injectable poly(lactic-co-glycolic acid) microsphere scaffold[J]. J Endod, 2017,43(5):745-750. |
| [15] | Chrepa V, Austah O, Diogenes A . Evaluation of a commercially available hyaluronic acid hydrogel (restylane) as injectable scaffold for dental pulp regeneration: an in vitro evaluation[J]. J Endod, 2017,43(2):257-262. |
| [16] | Yu L, Ding J . Injectable hydrogels as unique biomedical materials[J]. Chem Soc Rev, 2008,37(8):1473. |
| [17] | Malda J, Visser J, Melchels FP , et al. 25th anniversary article: engineering hydrogels for biofabrication[J]. Adv Mater, 2013,25(36):5011-5028. |
| [18] | Smith LR, Cho S, Discher DE . Stem cell differentiation is regu-lated by extracellular matrix mechanics[J]. Physiology, 2018,33(1):16-25. |
| [19] | Sun TL, Kurokawa T, Kuroda S , et al. Physical hydrogels composed of polyampholytes demonstrate high toughness and viscoelasticity[J]. Nat Mater, 2013,12(10):932-937. |
| [20] | Nonoyama T, Wada S, Kiyama R , et al. Double-network hydrogels strongly bondable to bones by spontaneous osteogenesis penetration[J]. Adv Mater, 2016,28(31):6740-6745. |
| [21] | Haque MA, Kurokawa T, Gong JP . Super tough double network hydrogels and their application as biomaterials[J]. Polymer, 2012,53(9):1805-1822. |
| [22] | Bellamy C, Shrestha S, Torneck C , et al. Effects of a bioactive scaffold containing a sustained transforming growth factor-β1-re-leasing nanoparticle system on the migration and differentiation of stem cells from the apical papilla[J]. J Endod, 2016,42(9):1385-1392. |
| [23] | Galler KM, Cavender AC, Koeklue U , et al. Bioengineering of dental stem cells in a PE gylated fibrin gel[J]. Regen Med, 2011,6(2):191-200. |
| [24] | Vining KH, Mooney DJ . Mechanical forces direct stem cell be-haviour in development and regeneration[J]. Nat Rev Mol Cell Bio, 2017,18(12):728-742. |
| [25] | Caiazzo M, Okawa Y, Ranga A , et al. Defined three-dimensional microenvironments boost induction of pluripotency[J]. Nat Mater, 2016,15(3):344-352. |
| [26] | Duval K, Grover H, Han L , et al. Modeling physiological events in 2D vs. 3D cell culture[J]. Physiology, 2017,32(4):266-277. |
| [27] | Soares DG, Rosseto HL, Basso FG , et al. Chitosan-collagen biomembrane embedded with calcium-aluminate enhances dentinogenic potential of pulp cells[J]. Braz Oral Res, 2016,30(1):e54. |
| [28] | Galler KM, D Souza RN, Hartgerink JD , et al. Scaffolds for dental pulp tissue engineering[J]. Adv Dent Res, 2011,23(3):333-339. |
| [29] | Caliari SR, Burdick JA . A practical guide to hydrogels for cell culture[J]. Nat Methods, 2016,13(5):405-414. |
| [30] | Boland T, Mironov V, Gutowska A , et al. Cell and organ printing 2: Fusion of cell aggregates in three-dimensional gels[J]. Anat Rec Part A, 2003,272A(2):497-502. |
| [31] | Janmey PA, Miller RT . Mechanisms of mechanical signaling in development and disease[J]. J Cell Sci, 2011,124(1):9-18. |
| [32] | Chen CS, Mrksich M, Huang S , et al. Geometric control of cell life and death[J]. Science, 1997,276(5317):1425-1428. |
| [1] | Yu-ke LI,Mei WANG,Lin TANG,Yu-hua LIU,Xiao-ying CHEN. Effect of pH on the chelation between strontium ions and decellularized small intestinal submucosal sponge scaffolds [J]. Journal of Peking University (Health Sciences), 2023, 55(1): 44-51. |
| [2] | Yi DENG,Yi ZHANG,Bo-wen LI,Mei WANG,Lin TANG,Yu-hua LIU. Effects of different crosslinking treatments on the properties of decellularized small intestinal submucosa porous scaffolds [J]. Journal of Peking University (Health Sciences), 2022, 54(3): 557-564. |
| [3] | Qian-li ZHANG,Chong-yang YUAN,Li LIU,Shi-peng WEN,Xiao-yan WANG. Effects of electrospun collagen nanofibrous matrix on the biological behavior of human dental pulp cells [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 28-34. |
| [4] | Rong LI,Ke-long CHEN,Yong WANG,Yun-song LIU,Yong-sheng ZHOU,Yu-chun SUN. Establishment of a 3D printing system for bone tissue engineering scaffold fabrication and the evaluation of its controllability over macro and micro structure precision [J]. Journal of Peking University(Health Sciences), 2019, 51(1): 115-119. |
| [5] | LIU Yan, FU Yu, LIU Shuai, ZHOU Yan-heng. Effects of microstructure of mineralized collagen scaffolds on cell morphology of MG 63 [J]. Journal of Peking University(Health Sciences), 2014, 46(1): 19-24. |
|
||