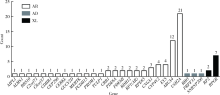
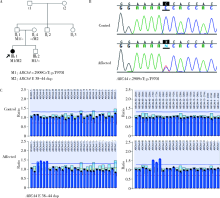
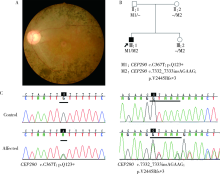
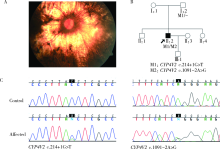
Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (5): 836-844. doi: 10.19723/j.issn.1671-167X.2020.05.007
Previous Articles Next Articles
Comparison study of whole exome sequencing and targeted panel sequencing in molecular diagnosis of inherited retinal dystrophies
Xiao-zhen LIU,Ying-ying LI,Li-ping YANG( )
)
- Department of Ophthalmology, Peking University Third Hospital, Beijing 100191, China
CLC Number:
- R774.13
| [1] |
Berger W, Kloeckener-Gruissem B, Neidhardt J. The molecular basis of human retinal and vitreoretinal diseases[J]. Prog Retin Eye Res, 2010,29(5):335-375.
doi: 10.1016/j.preteyeres.2010.03.004 |
| [2] |
Zhao L, Wang F, Wang H, et al. Next-generation sequencing-based molecular diagnosis of 82 retinitis pigmentosa probands from Northern Ireland[J]. Hum Genet, 2015,134(2):217-230.
doi: 10.1007/s00439-014-1512-7 pmid: 25472526 |
| [3] |
Glockle N, Konl LS, Mohr J, et al. Panel-based next generation sequencing as a reliable and efficient technique to detect mutations in unselected patients with retinal dystrophies[J]. Eur J Hum Genet, 2014,22(1):99-104.
doi: 10.1038/ejhg.2013.72 |
| [4] | Beryozkin A, Shevah E, Kimchi A, et al. Whole exome sequencing reveals mutations in known retinal disease genes in 33 out of 68 Israeli families with inherited retinopathies[J]. Sci Rep, 2015(5):13187. |
| [5] |
Yang L, Cui H, Yin X, et al. Dependable and efficient clinical molecular diagnosis of Chinese RP patient with targeted exon sequencing[J]. PLoS One, 2015,10(10):e0140684.
doi: 10.1371/journal.pone.0140684 pmid: 26496393 |
| [6] | Riera M, Navarro R, Ruiz-Nogales S, et al. Whole exome sequencing using Ion Proton system enables reliable genetic diagnosis of inherited retinal dystrophies[J]. Sci Rep, 2017(7):42078. |
| [7] |
Zhang J, Wang C, Shen Y, et al. A mutation in ADIPOR1 causes nonsyndromic autosomal dominant retinitis pigmentosa[J]. Hum Genet, 2016,135(12):1375-1387.
doi: 10.1007/s00439-016-1730-2 pmid: 27655171 |
| [8] |
Bader I, Brandau O, Achatz H, et al. X-linked retinitis pigmentosa: RPGR mutations in most families with definite X linkage and clustering of mutations in a short sequence stretch of exon ORF15[J]. Invest Ophthalmol Vis Sci, 2003,44(4):1458-1463.
doi: 10.1167/iovs.02-0605 pmid: 12657579 |
| [9] |
Li L, Xiao X, Li S, et al. Detection of variants in 15 genes in 87 unrelated Chinese patients with Leber congenital amaurosis[J]. PLoS One, 2011,6(5):e19458.
doi: 10.1371/journal.pone.0019458 pmid: 21602930 |
| [10] |
Xiao X, Mai G, Li S, et al. Identification of CYP4V2 mutation in 21 families and overview of mutation spectrum in Bietti crystalline corneoretinal dystrophy[J]. Biochem Biophys Res Commun, 2011,409(2):181-186.
doi: 10.1016/j.bbrc.2011.04.112 pmid: 21565171 |
| [11] |
Li A, Jiao X, Munier FL, et al. Bietti crystalline corneoretinal dystrophy is caused by mutations in the novel gene CYP4V2[J]. Am J Hum Genet, 2004,74(5):817-826.
doi: 10.1086/383228 |
| [12] |
Consugar MB, Navarro-Gomez D, Place EM, et al. Panel-based genetic diagnostic testing for inherited eye diseases is highly accurate and reproducible, and more sensitive for variant detection, than exome sequencing[J]. Genet Med, 2015,17(4):253-261.
doi: 10.1038/gim.2014.172 pmid: 25412400 |
| [13] |
Bujakowska KM, Fernandez-Godino R, Place E, et al. Copy-number variation is an important contributor to the genetic causality of inherited retinal degenerations[J]. Genet Med, 2017,19(6):643-651.
doi: 10.1038/gim.2016.158 pmid: 27735924 |
| [14] |
Haer-Wigman L, van Zelst-Stams WA, Pfundt R, et al. Diagnostic exome sequencing in 266 Dutch patients with visual impairment[J]. Eur J Hum Genet, 2017,25(5):591-599.
doi: 10.1038/ejhg.2017.9 pmid: 28224992 |
| [15] | Broadgate S, Yu J, Downes SM, et al. Unravelling the genetics of inherited retinal dystrophies: Past, present and future[J]. Prog Retin Eye Res, 2017(59):53-96. |
| [16] | 周雪莹, 于志强. 全基因组外显子测序在眼科遗传病中的应用[J]. 中华眼科杂志, 2015,51(5):395-400. |
| [17] |
ACMG Board of Directors Points to consider in the clinical application of genomic sequencing[J]. Genet Med, 2012,14(8):759-761.
doi: 10.1038/gim.2012.74 pmid: 22863877 |
| [18] |
Yuzawa M, Mae Y, Matsui M. Bietti’s crystalline retinopathy[J]. Ophthalmic Paediatr Genet, 1986,7(1):9-20.
doi: 10.3109/13816818609058037 pmid: 3703493 |
| [19] |
Lee KY, Koh AH, Aung T, et al. Characterization of Bietti crystalline dystrophy patients with CYP4V2 mutations[J]. Invest Ophthalmol Vis Sci, 2005,46(10):3812-3816.
pmid: 16186368 |
| [20] |
Tiwari A, Lemke J, Altmueller J, et al. Identification of novel and recurrent disease-causing mutations in retinal dystrophies using whole exome sequencing (WES): Benefits and limitations[J]. PLoS One, 2016,11(7):e0158692.
doi: 10.1371/journal.pone.0158692 pmid: 27391102 |
| [1] | Shang XIE,Zhi-gang CAI,Xiao-feng SHAN. Application value of whole exon sequencing and immune related indicators in the precision treatment of oral squamous cell carcinoma [J]. Journal of Peking University (Health Sciences), 2023, 55(4): 697-701. |
| [2] | Ya-fei LIU,Lin-lin SONG,Mao-wei XING,Li-xin CAI,Dong-xin WANG. Comparison of pulse pressure variation, stroke volume variation, and plethysmographic variability index in pediatric patients undergoing craniotomy [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 946-951. |
| [3] | FENG Ke,NI Jing-jing,XIA Yan-qing,QU Xiao-wei,ZHANG Hui-juan,WAN Feng,HONG Kai,ZHANG Cui-lian,GUO Hai-bin. Genetic analysis of three cases of acephalic spermatozoa syndrome caused by SUN5 mutation and the outcome of assisted reproductive technology [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 803-807. |
| [4] | WU Kai1 ZHANG Yang, ZHANG Hong, TAN Zeng-huan2 GUO Xiao-hui, YANG Jian-mei. Germline gene testing of the RET, VHL, SDHD and SDHB genes in patients with pheochromocytoma/paraganglioma [J]. Journal of Peking University(Health Sciences), 2018, 50(4): 634-639. |
| [5] | XIE Gao-Qiang-1△, YU Hui-2, CHEN Jing-Zhou-2, ZHAO Lian-Cheng-3△, REN Fu-Xiu-4, SHI Ping-4, WU Yang-Feng-1, 5 , 6 . Relationship of genetic variants and cardiovascular risk factors with interleukin-6 and interleukin-10 secreted by monocytes [J]. Journal of Peking University(Health Sciences), 2014, 46(4): 589-595. |
| [6] | ZHANG Jia-ni, SHI Tie-mei. Reliability of antral follicle counts using transvaginal two- and three- dimensional sonography [J]. Journal of Peking University(Health Sciences), 2013, 45(6): 896-900. |
| [7] | ZHEN Xiu-mei, SUN Yi-min, QIAO Jie, LI Rong, WANG Li-na, LIU Ping . Genome-wide copy number scan in Chinese patients with premature ovarian failure [J]. Journal of Peking University(Health Sciences), 2013, 45(6): 841-847. |
|
||