Journal of Peking University (Health Sciences) ›› 2020, Vol. 52 ›› Issue (5): 856-862. doi: 10.19723/j.issn.1671-167X.2020.05.010
Previous Articles Next Articles
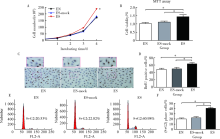
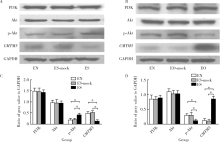
Association of CMTM5 gene expression with the risk of in-stent restenosis in patients with coronary artery disease after drug-eluting stent implantation and the effects and mechanisms of CMTM5 on human vascular endothelial cells
Teng-fei LIU,Tao LIN,Li-hui REN,Guang-ping LI,Jian-jun PENG( )
)
- Department of Cardiology, Shijitan Hospital, Beijing 100034, China
CLC Number:
- R543.3
| [1] |
Kokkinidis DG, Waldo SW, Armstrong EJ. Treatment of coronary artery in-stent restenosis[J]. Expert Rev Cardiovasc Ther, 2017,15(3):191-202.
doi: 10.1080/14779072.2017.1284588 pmid: 28116914 |
| [2] |
Kuchulakanti PK, Chu WW, Torguson R, et al. Correlates and long-term outcomes of angiographically proven stent thrombosis with sirolimus- and paclitaxel-eluting stents[J]. Circulation, 2006,113(8):1108-1113.
doi: 10.1161/CIRCULATIONAHA.105.600155 pmid: 16490815 |
| [3] |
Philip F. Duration of triple therapy in patients requiring oral anticoagulation after drug-eluting stent implantation[J]. J Am Coll Cardiol, 2015,66(9):1088-1089.
pmid: 26314542 |
| [4] |
Byrne RA, Joner M, Kastrati A. Stent thrombosis and restenosis: what have we learned and where are we going? The andreas gruntzig lecture ESC 2014[J]. Eur Heart J, 2015,36(47):3320-3331.
doi: 10.1093/eurheartj/ehv511 pmid: 26417060 |
| [5] |
Moliterno DJ. Healing achilles: sirolimus versus paclitaxel[J]. N Engl J Med, 2005,353(7):724-727.
doi: 10.1056/NEJMe058140 pmid: 16105991 |
| [6] |
Virmani R, Farb A. Pathology of in-stent restenosis[J]. Curr Opin Lipidol, 1999,10(6):499-506.
doi: 10.1097/00041433-199912000-00004 pmid: 10680043 |
| [7] |
Ma X, Hibbert B, McNulty M, et al. Heat shock protein 27 attenuates neointima formation and accelerates reendothelialization after arterial injury and stent implantation: importance of vascular endothelial growth factor up-regulation[J]. FASEB J, 2014,28(2):594-602.
doi: 10.1096/fj.13-230417 |
| [8] |
Li H, Guo X, Shao L, et al. CMTM5-v1, a four-transmembrane protein, presents a secreted form released via a vesicle-mediated secretory pathway[J]. BMB Rep, 2010,43(3):182-187.
doi: 10.5483/bmbrep.2010.43.3.182 pmid: 20356458 |
| [9] |
Voora D, Cyr D, Lucas J, et al. Aspirin exposure reveals novel genes associated with platelet function and cardiovascular events[J]. J Am Coll Cardiol, 2013,62(14):1267-1276.
doi: 10.1016/j.jacc.2013.05.073 pmid: 23831034 |
| [10] |
Liu TF, Zhang JW, Chen XH, et al. Comparison between urinary 11-dehydrothromboxane B2 detection and platelet light transmission aggregometry (LTA) assays for evaluating aspirin response in elderly patients with coronary artery disease[J]. Gene, 2015,571(1):23-27.
doi: 10.1016/j.gene.2015.06.045 pmid: 26095809 |
| [11] |
刘滕飞, 张婧薇, 陈夏欢, 等. CMTM5基因rs723840单核苷酸多态性与阿司匹林治疗下血小板高反应性的相关性研究[J]. 北京大学学报(医学版), 2015,47(6):905-909.
doi: 10.3969/j.issn.1671167X.2015.06.003 |
| [12] |
Teirstein P, Reilly JP. Late stent thrombosis in brachytherapy: the role of long-term antiplatelet therapy[J]. J Invasive Cardiol, 2002,14(3):109-114.
pmid: 11870263 |
| [13] | Schmieder RE. Endothelial dysfunction: how can one intervene at the beginning of the cardiovascular continuum[J]. J Hypertens Suppl, 2006,24(Suppl 2):S31-35. |
| [14] |
Xiao Y, Yuan Y, Zhang Y, et al. CMTM5 is reduced in prostate cancer and inhibits cancer cell growth in vitro and in vivo[J]. Clin Transl Oncol, 2015,17(6):431-437.
doi: 10.1007/s12094-014-1253-z pmid: 25387568 |
| [1] | ZHUANG Jin-man,LI Tian-run,LI Xuan,LUAN Jing-yuan,WANG Chang-ming,FENG Qi-chen,HAN Jin-tao. Application of Rotarex mechanical thrombectomy system in treating in-stent restenosis of lower extremity arteriosclerosis obliterans [J]. Journal of Peking University (Health Sciences), 2021, 53(4): 740-743. |
|
||