Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (3): 421-426. doi: 10.19723/j.issn.1671-167X.2022.03.005
Previous Articles Next Articles
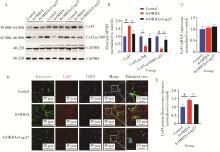
Inhibition connexin 43 by mimetic peptide Gap27 mediates protective effects on 6-hydroxydopamine induced Parkinson's disease mouse model
Hui-hui QUAN,Wei-xing XU,Yu-ze QI,Qing-ru LI,Hui ZHOU,Jing HUANG*( )
)
- Department of Occupational and Environmental Health, Peking University School of Public Health, Beijing 100191, China
CLC Number:
- R392.3
| 1 |
Hayes MT . Parkinson's disease and Parkinsonism[J]. Am J Med, 2019, 132 (7): 802- 807.
doi: 10.1016/j.amjmed.2019.03.001 |
| 2 |
Luo H , Xiang Y , Qu X , et al. Apelin-13 suppresses neuroinflammation against cognitive deficit in a streptozotocin-induced rat mo-del of Alzheimer's disease through activation of BDNF-TrkB signaling pathway[J]. Front Pharmacol, 2019, 10, 395.
doi: 10.3389/fphar.2019.00395 |
| 3 | 苏晓梅, 张丹参. 星形胶质细胞与神经退行性疾病的相关性[J]. 中国药理学与毒理学杂志, 2019, 33 (10): 868- 869. |
| 4 |
Phatnani H , Maniatis T . Astrocytes in neurodegenerative disease[J]. Cold Spring Harb Perspect Biol, 2015, 7 (6): e020628.
doi: 10.1101/cshperspect.a020628 |
| 5 | Diaz EF , Labra VC , Alvear TF , et al. Connexin 43 hemichannels and pannexin-1 channels contribute to the α-synuclein-induced dysfunction and death of astrocytes[J]. Glia, 2019, 67 (8): 1598- 1619. |
| 6 |
Orellana JA , Sáez PJ , Cortés-Campos C , et al. Glucose increases intracellular free Ca(2+) in tanycytes via ATP released through connexin 43 hemichannels[J]. Glia, 2012, 60 (1): 53- 68.
doi: 10.1002/glia.21246 |
| 7 |
Leithe E , Mesnil M , Aasen T . The connexin 43 C-terminus: a tail of many tales[J]. Biochim Biophys Acta Biomembr, 2018, 1860 (1): 48- 64.
doi: 10.1016/j.bbamem.2017.05.008 |
| 8 | Evans WH , Boitano S . Connexin mimetic peptides: specific inhi-bitors of gap-junctional intercellular communication[J]. Biochem Soc Trans, 2001, 29 (Pt 4): 606- 612. |
| 9 |
Li X , Zhao H , Tan X , et al. Inhibition of connexin43 improves functional recovery after ischemic brain injury in neonatal rats[J]. Glia, 2015, 63 (9): 1553- 1567.
doi: 10.1002/glia.22826 |
| 10 | Faniku C , O'Shaughnessy E , Lorraine C , et al. The connexin mimetic peptide gap27 and Cx43-knockdown reveal differential roles for connexin43 in wound closure events in skin model systems[J]. Int J Mol Sci, 2018, 9 (2): 604. |
| 11 |
Evans WH , Leybaert L . Mimetic peptides as blockers of connexin channel-facilitated intercellular communication[J]. Cell Commun Adhes, 2007, 14 (6): 265- 273.
doi: 10.1080/15419060801891034 |
| 12 |
Solan JL , Lampe PD . Connexin43 phosphorylation: structural changes and biological effects[J]. Biochem J, 2009, 419 (2): 261- 272.
doi: 10.1042/BJ20082319 |
| 13 |
Ek-Vitorin JF , King TJ , Heyman NS , et al. Selectivity of conne-xin 43 channels is regulated through protein kinase C-dependent phosphorylation[J]. Circ Res, 2006, 98 (12): 1498- 1505.
doi: 10.1161/01.RES.0000227572.45891.2c |
| 14 | 黄焕焕, 赵雨佳, 张慧峰, 等. 抑制星形胶质细胞Cx43可保护LPS诱导的多巴胺神经元损伤[J]. 毒理学杂志, 2018, 32 (3): 186- 194. |
| 15 |
Wang ZY , Lian H , Zhou L , et al. Altered expression of d1 and d2 dopamine receptors in vagal neurons innervating the gastric muscularis externa in a Parkinson's disease rat model[J]. J Parkinsons Dis, 2016, 6 (2): 317- 323.
doi: 10.3233/JPD-160817 |
| 16 | 张蓓, 淫羊藿苷对6-OHDA诱导的帕金森病小鼠模型的保护作用及机制研究[D]. 贵州: 遵义医科大学, 2019. |
| 17 | Franklin-Keith BJ , Paxinos G . The mouse brain in stereotaxic coordinates[M]. 3th ed New York: Academic Press, 2007: 360 |
| 18 |
Cotter ML , Boitano S , Lampe PD , et al. The lipidated connexin mimetic peptide SRPTEKT-Hdc is a potent inhibitor of Cx43 channels with specificity for the pS368 phospho-isoform[J]. Am J Physiol, 2019, 317 (4): C825- C842.
doi: 10.1152/ajpcell.00160.2019 |
| 19 |
Rufer M , Wirth SB , Hofer A , et al. Regulation of connexin-43, GFAP, and FGF-2 is not accompanied by changes in astroglial coupling in MPTP-lesioned, FGF-2-treated Parkinsonian mice[J]. J Neurosci Res, 1996, 46 (5): 606- 617.
doi: 10.1002/(SICI)1097-4547(19961201)46:5<606::AID-JNR9>3.0.CO;2-N |
| 20 |
Kawasaki A , Hayashi T , Nakachi K , et al. Modulation of conne-xin 43 in rotenone-induced model of Parkinson's disease[J]. Neuroscience, 2009, 160 (1): 61- 68.
doi: 10.1016/j.neuroscience.2009.01.080 |
| 21 |
Wang Y , Wu Z , Liu X , et al. Gastrodin ameliorates Parkinson's disease by downregulating connexin 43[J]. Mol Med Rep, 2013, 8 (2): 585- 590.
doi: 10.3892/mmr.2013.1535 |
| 22 |
韩雪洁, 哈力达·巴合提汗, 高华, 等. 黄芩苷可影响帕金森病模型大鼠纹状体星形胶质细胞缝隙连接蛋白43的表达[J]. 中国组织工程研究, 2018, 22 (16): 2542- 2548.
doi: 10.3969/j.issn.2095-4344.0222 |
| 23 |
Epifantseva I , Shaw RM . Intracellular trafficking pathways of Cx43 gap junction channels[J]. Biochim Biophys Acta Biomembr, 2018, 1860 (1): 40- 47.
doi: 10.1016/j.bbamem.2017.05.018 |
| 24 | Charron G , Doudnikoff E , Canron MH , et al. Astrocytosis in Parkinsonism: considering tripartite striatal synapses in physiopathology?[J]. Front Aging Neurosci, 2014, 6, 1- 12. |
| No related articles found! |
|
||