Journal of Peking University (Health Sciences) ›› 2024, Vol. 56 ›› Issue (6): 963-971. doi: 10.19723/j.issn.1671-167X.2024.06.004
Previous Articles Next Articles
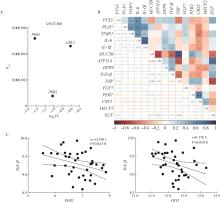
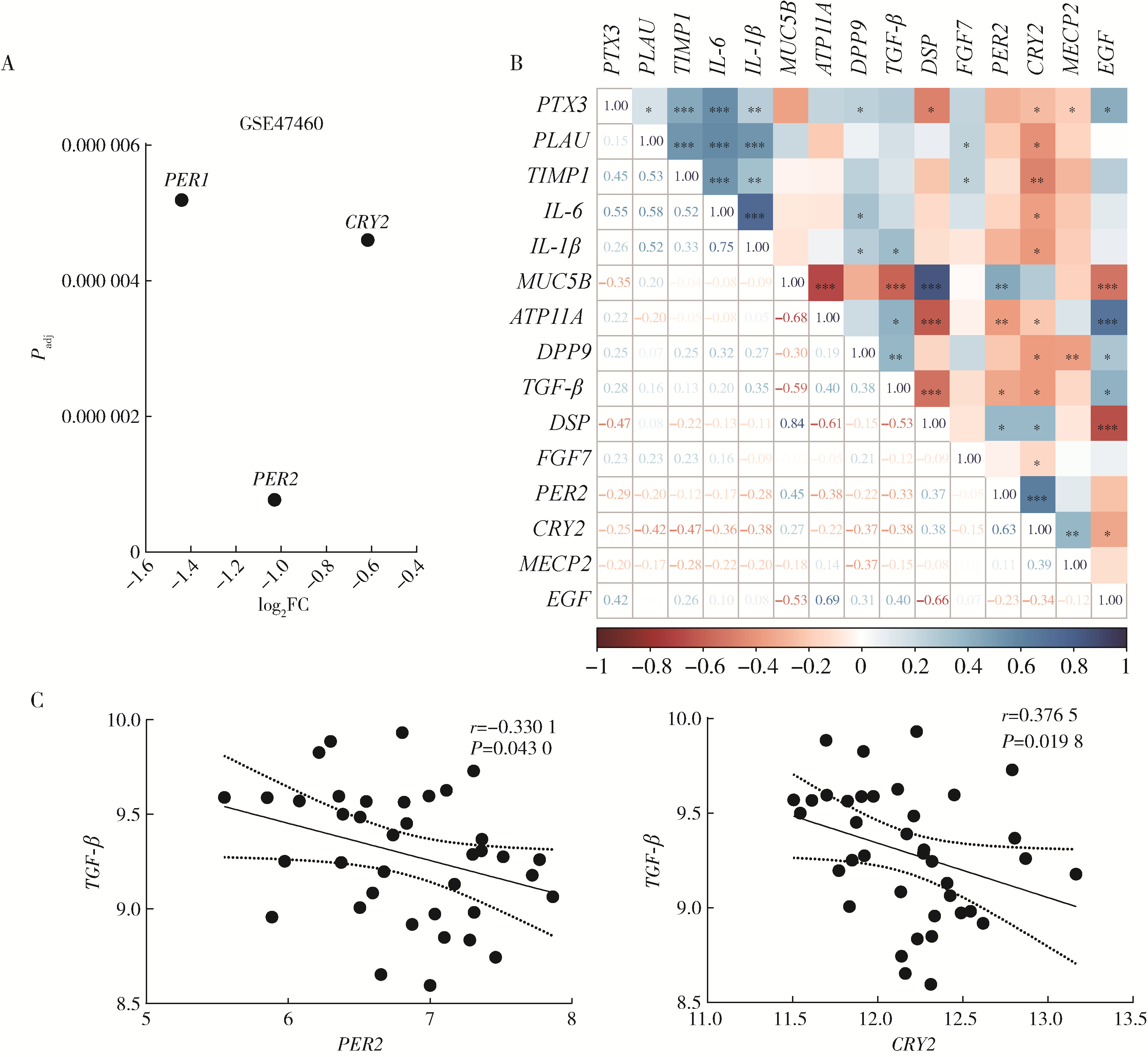
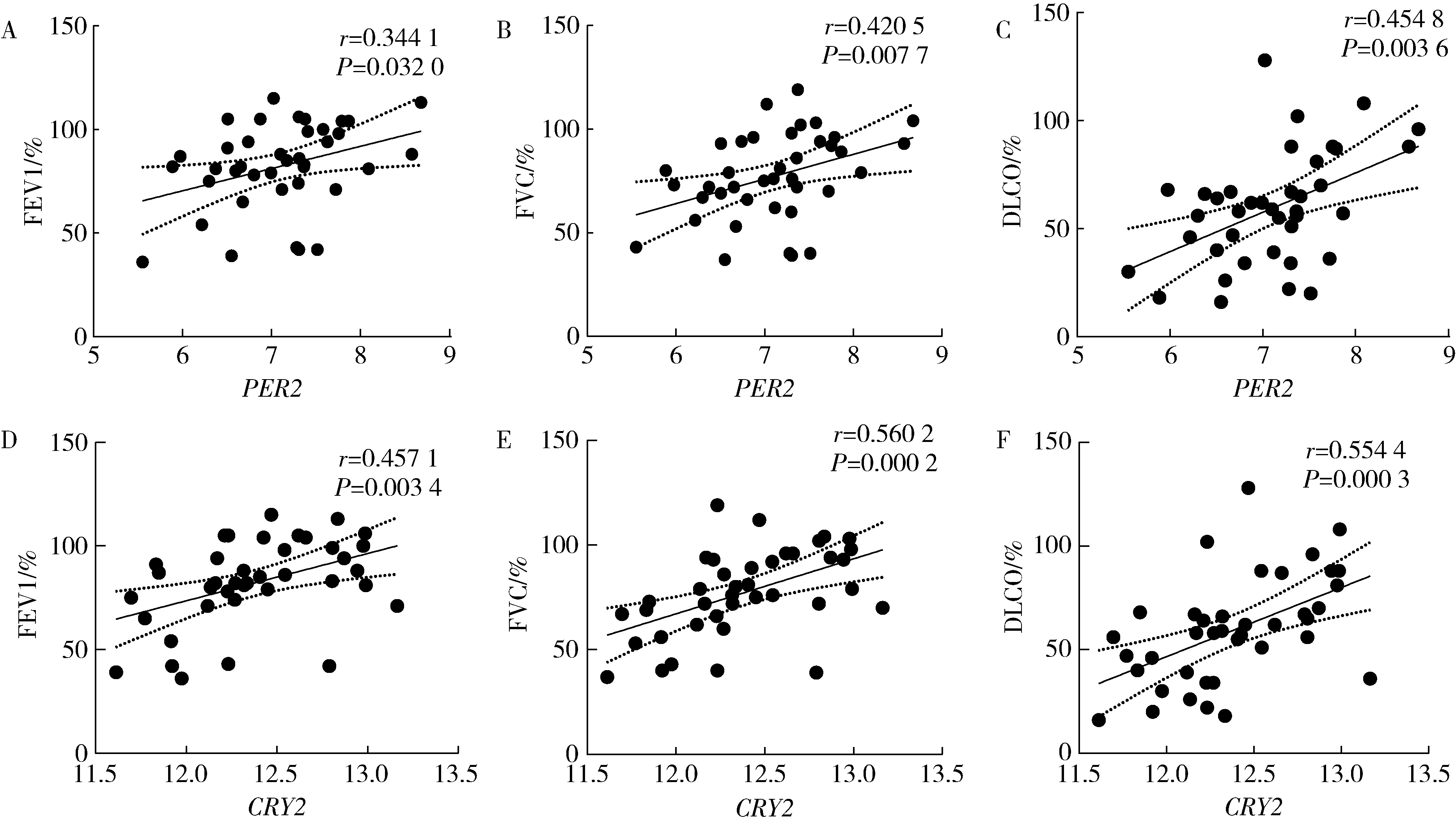
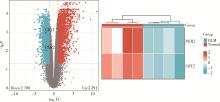
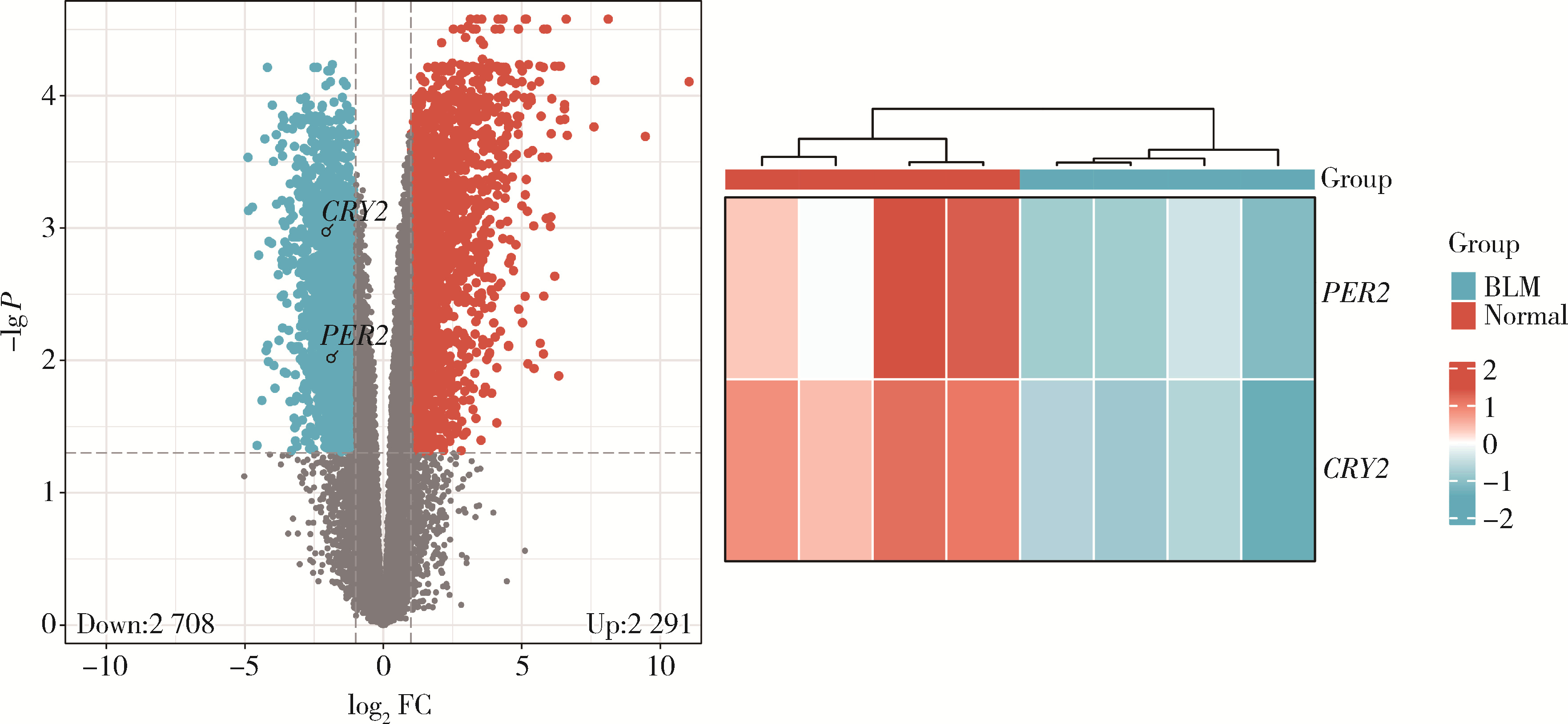
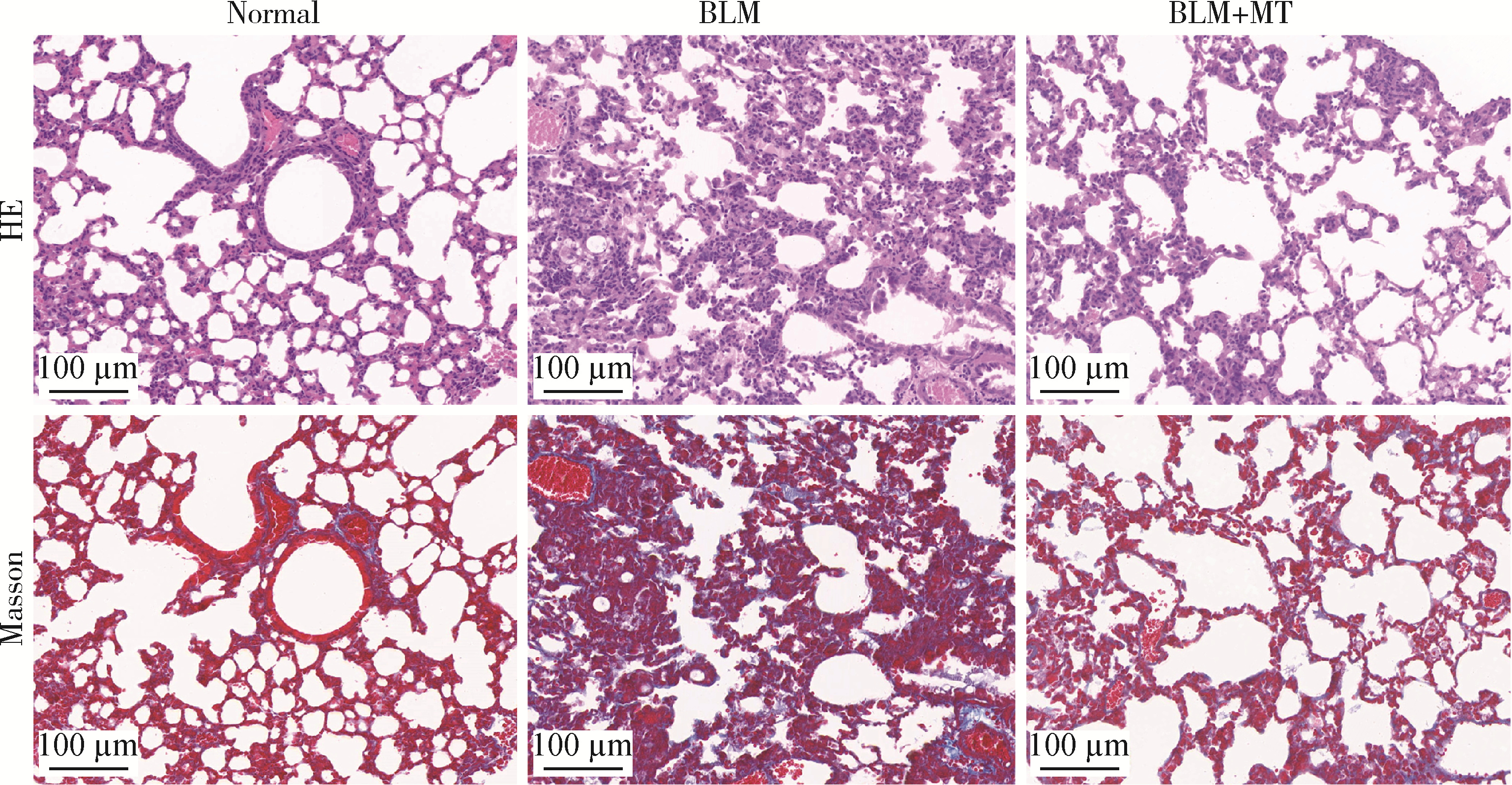
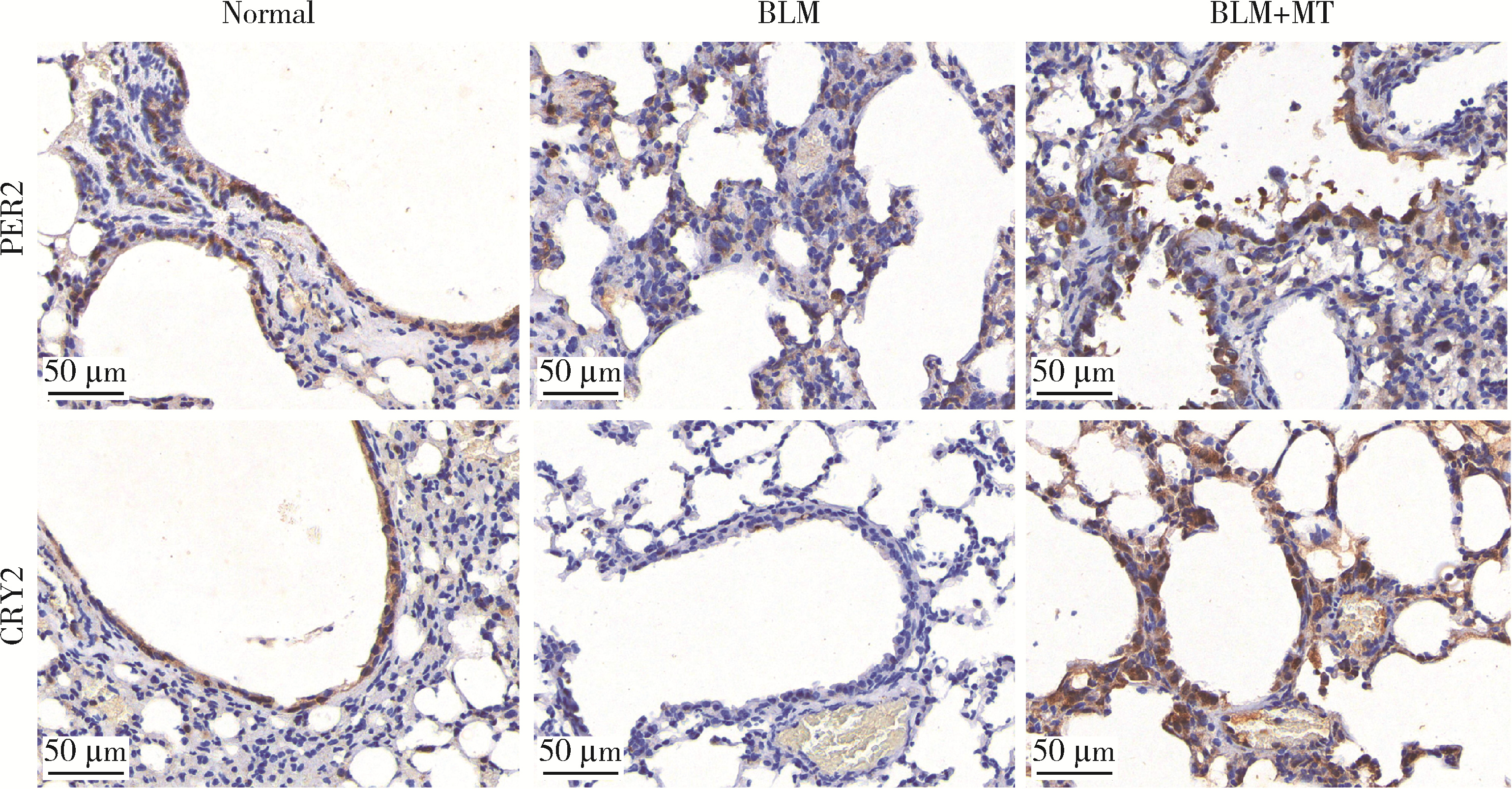
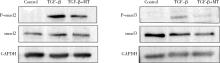
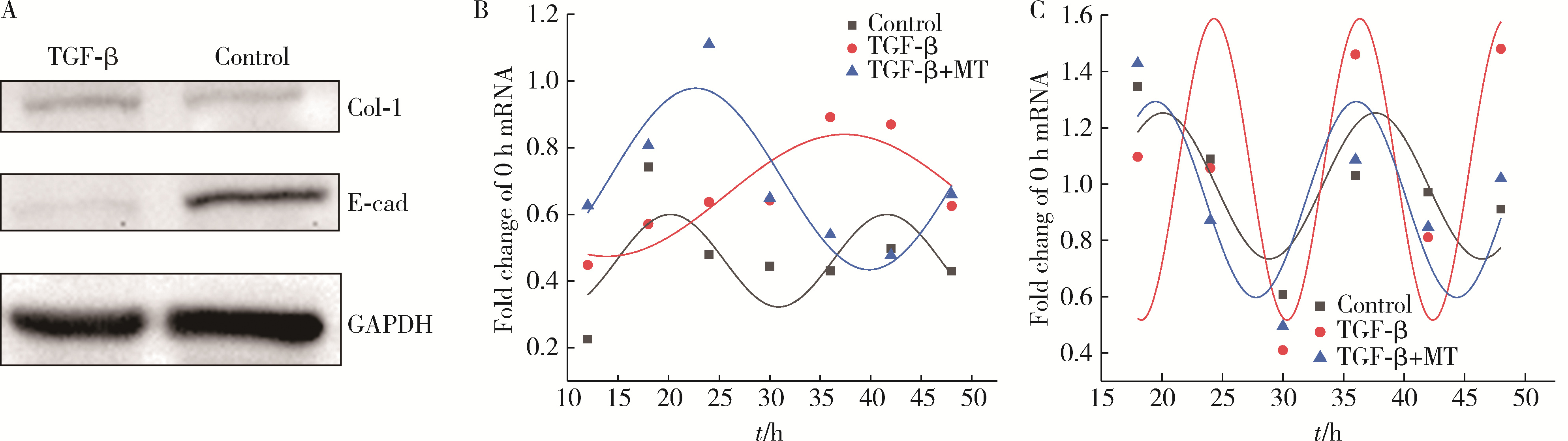
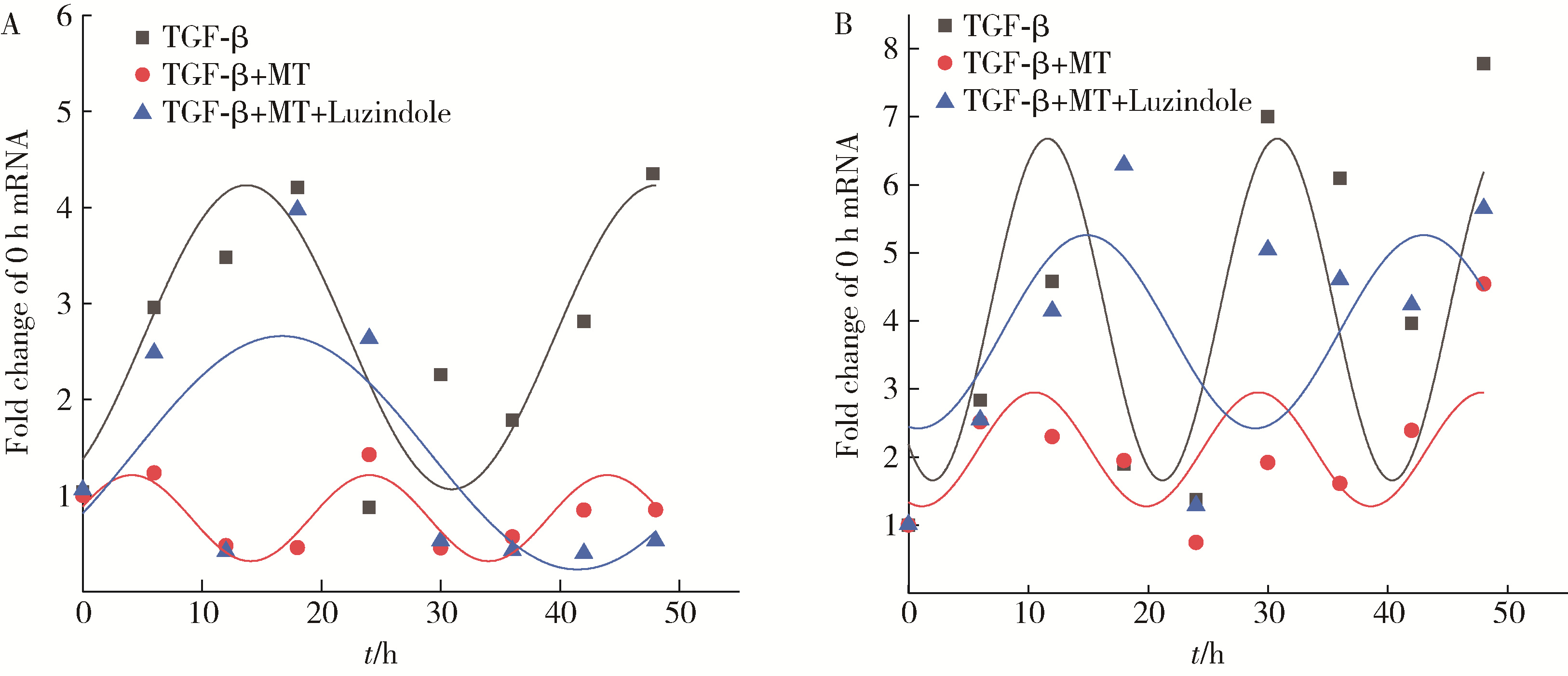
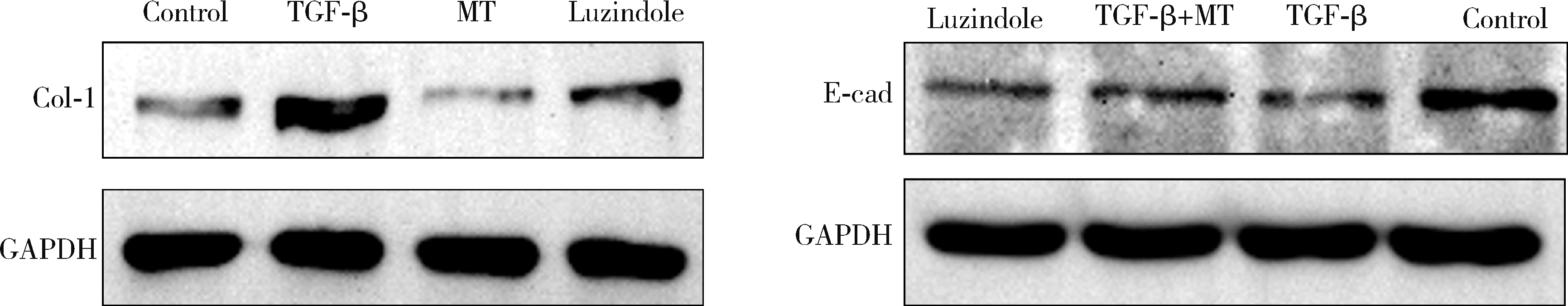
Mechanism of melatonin regulating the expression level of rhythm genes to alleviate interstitial pulmonary fibrosis
Bingle LI1, Lingyan ZHU2, Yongfu WANG2,*( ), Li BAI3,4,*(
), Li BAI3,4,*( )
)
- 1. Baotou Medical College, Inner Mongolia University of Science and Technology, Baotou 014010, Inner Mongolia, China
2. Department of Rheumatology and Immunology, the First Affiliated Hospital of Baotou Medical College, Baotou 014010, Inner Mongolia, China
3. The Central Lab, the First Affiliated Hospital of Baotou Medical College, Baotou 014010, Inner Mongolia, China
4. Inner Mongolia Autoimmune Key Laboratory, Baotou 014010, Inner Mongolia, China
CLC Number:
- R563
| 1 |
Koike N , Yoo SH , Huang HC , et al. Transcriptional architecture and chromatin landscape of the core circadian clock in mammals[J]. Science, 2012, 338 (6105): 349- 354.
doi: 10.1126/science.1226339 |
| 2 |
Yamazaki S , Numano R , Abe M , et al. Resetting central and peripheral circadian oscillators in transgenic rats[J]. Science, 2000, 288 (5466): 682- 685.
doi: 10.1126/science.288.5466.682 |
| 3 |
Cox KH , Takahashi JS . Circadian clock genes and the transcriptional architecture of the clock mechanism[J]. J Mol Endocrinol, 2019, 63 (4): R93- R102.
doi: 10.1530/JME-19-0153 |
| 4 |
Fukuhara C , Tosini G . Peripheral circadian oscillators and their rhythmic regulation[J]. Front Biosci, 2003, 8, d642- d651.
doi: 10.2741/1042 |
| 5 | Oishi A , Jockers R . Measuring protein-protein interactions of melatonin receptors by bioluminescence resonance energy transfer (BRET)[J]. Methods Mol Biol, 2022, 2550, 207- 218. |
| 6 |
Cipolla-Neto J , Amaral FGD . Melatonin as a hormone: New phy-siological and clinical insights[J]. Endocr Rev, 2018, 39 (6): 990- 1028.
doi: 10.1210/er.2018-00084 |
| 7 |
Kandalepas PC , Mitchell JW , Gillette MU . Melatonin signal transduction pathways require E-box-mediated transcription of Per1 and Per2 to reset the SCN Clock at dusk[J]. PLoS One, 2016, 11 (6): e0157824.
doi: 10.1371/journal.pone.0157824 |
| 8 |
Zhao SQ , Gao Y , Zhang Y , et al. cAMP/PKA/CREB signaling pathway-mediated effects of melatonin receptor genes on clock gene expression in Bactrian camel ovarian granulosa cells[J]. Domest Anim Endocrinol, 2021, 76, 106609.
doi: 10.1016/j.domaniend.2021.106609 |
| 9 |
Sisto M , Ribatti D , Lisi S . Organ fibrosis and autoimmunity: The role of inflammation in TGFbeta-dependent EMT[J]. Biomolecules, 2021, 11 (2): 310.
doi: 10.3390/biom11020310 |
| 10 |
Frangogiannis N . Transforming growth factor-beta in tissue fibrosis[J]. J Exp Med, 2020, 217 (3): e20190103.
doi: 10.1084/jem.20190103 |
| 11 |
Andugulapati SB , Gourishetti K , Tirunavalli SK , et al. Biochanin- A ameliorates pulmonary fibrosis by suppressing the TGF-beta mediated EMT, myofibroblasts differentiation and collagen deposition in in vitro and in vivo systems[J]. Phytomedicine, 2020, 78, 153298.
doi: 10.1016/j.phymed.2020.153298 |
| 12 |
Cunningham PS , Meijer P , Nazgiewicz A , et al. The circadian clock protein REVERBα inhibits pulmonary fibrosis development[J]. Proc Natl Acad Sci USA, 2020, 117 (2): 1139- 1147.
doi: 10.1073/pnas.1912109117 |
| 13 |
Chen SJ , Yu F , Feng X , et al. DEC1 is involved in circadian rhythm disruption-exacerbated pulmonary fibrosis[J]. Cell Commun Signal, 2024, 22 (1): 245.
doi: 10.1186/s12964-024-01614-w |
| 14 |
Dong C , Gongora R , Sosulski ML , et al. Regulation of transforming growth factor-beta1 (TGF-beta1)-induced pro-fibrotic activities by circadian clock gene BMAL1[J]. Respir Res, 2016, 17, 4.
doi: 10.1186/s12931-016-0320-0 |
| 15 |
Thannickal VJ , Toews GB , White ES , et al. Mechanisms of pulmonary fibrosis[J]. Annu Rev Med, 2004, 55, 395- 417.
doi: 10.1146/annurev.med.55.091902.103810 |
| 16 |
Inui N , Sakai S , Kitagawa M . Molecular pathogenesis of pulmonary fibrosis, with focus on pathways related to TGF-beta and the Ubiquitin-Proteasome pathway[J]. Int J Mol Sci, 2021, 22 (11): 6107.
doi: 10.3390/ijms22116107 |
| 17 |
Chanda D , Otoupalova E , Smith SR , et al. Developmental pathways in the pathogenesis of lung fibrosis[J]. Mol Aspects Med, 2019, 65, 56- 69.
doi: 10.1016/j.mam.2018.08.004 |
| 18 |
Rodriguez-Santana C , Lopez-Rodriguez A , Martinez-Ruiz L , et al. The relationship between Clock genes, sirtuin 1, and mitochondrial activity in head and neck squamous cell cancer: Effects of melatonin treatment[J]. Int J Mol Sci, 2023, 24 (19): 15030.
doi: 10.3390/ijms241915030 |
| 19 |
Shin NR , Park JW , Lee IC , et al. Melatonin suppresses fibrotic responses induced by cigarette smoke via downregulation of TGF-beta1[J]. Oncotarget, 2017, 8 (56): 95692- 95703.
doi: 10.18632/oncotarget.21680 |
| 20 |
Li Y , Zhou Y , Zhao C , et al. The circadian clock gene, BMAL1, promotes radiosensitization in nasopharyngeal carcinoma by inhibiting the epithelial-to-mesenchymal transition via the TGF-beta1/Smads/Snail1 axis[J]. Oral Oncol, 2024, 152, 106798.
doi: 10.1016/j.oraloncology.2024.106798 |
| 21 |
Guo SN , Jiang XQ , Chen N , et al. Melatonin regulates circadian clock proteins expression in allergic airway inflammation[J]. Heliyon, 2024, 10 (6): e27471.
doi: 10.1016/j.heliyon.2024.e27471 |
| 22 |
Wan L , Shi XY , Ge WR , et al. The Instigation of the associations between melatonin, Circadian genes, and epileptic spasms in infant rats[J]. Front Neurol, 2020, 11, 497225.
doi: 10.3389/fneur.2020.497225 |
| 23 |
Fernandez-Ortiz M , Sayed RKA , Roman-Montoya Y , et al. Age and chronodisruption in mouse heart: Effect of the NLRP3 Inflammasome and melatonin therapy[J]. Int J Mol Sci, 2022, 23 (12): 6846.
doi: 10.3390/ijms23126846 |
| [1] | Shan HE,Xin CHEN,Qi CHENG,Lingjiang ZHU,Peiyu ZHANG,Shuting TONG,Jing XUE,Yan DU. Tofacitinib inhibits the transformation of lung fibroblasts into myofibroblasts through JAK/STAT3 pathway [J]. Journal of Peking University (Health Sciences), 2024, 56(3): 505-511. |
|
||