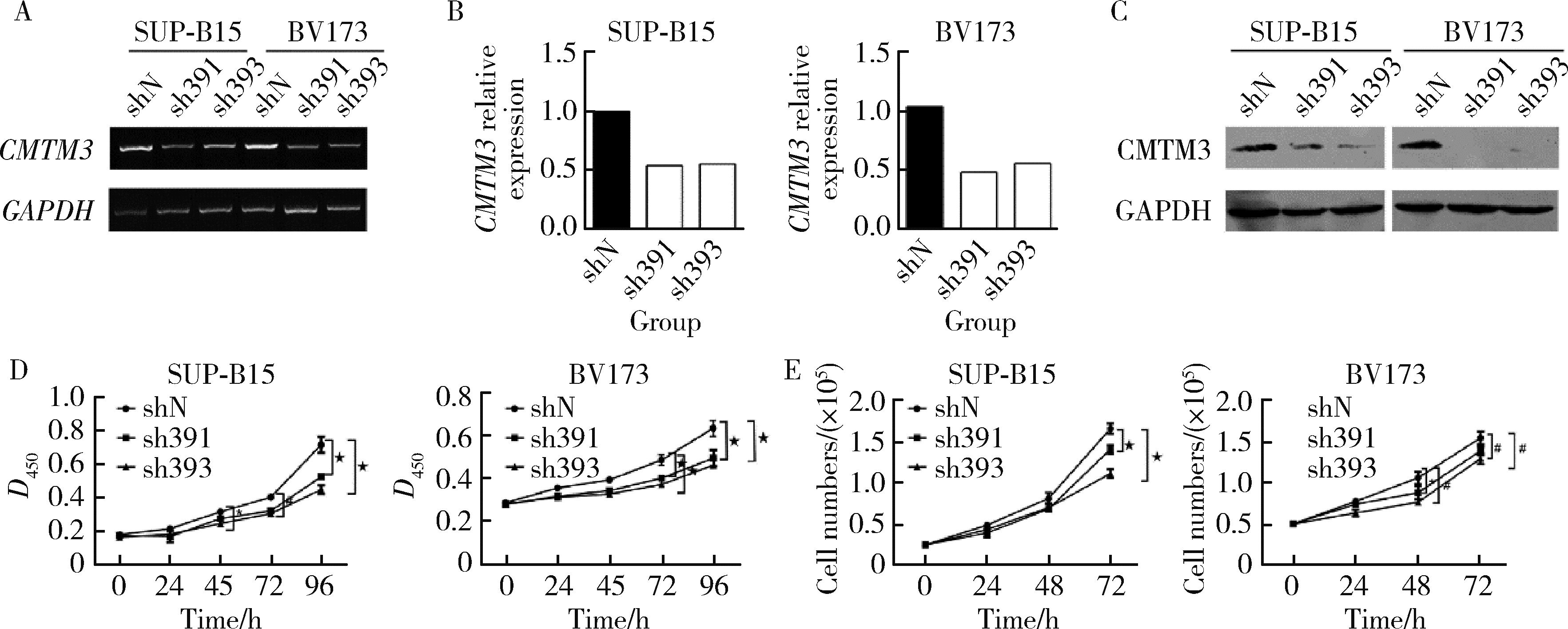
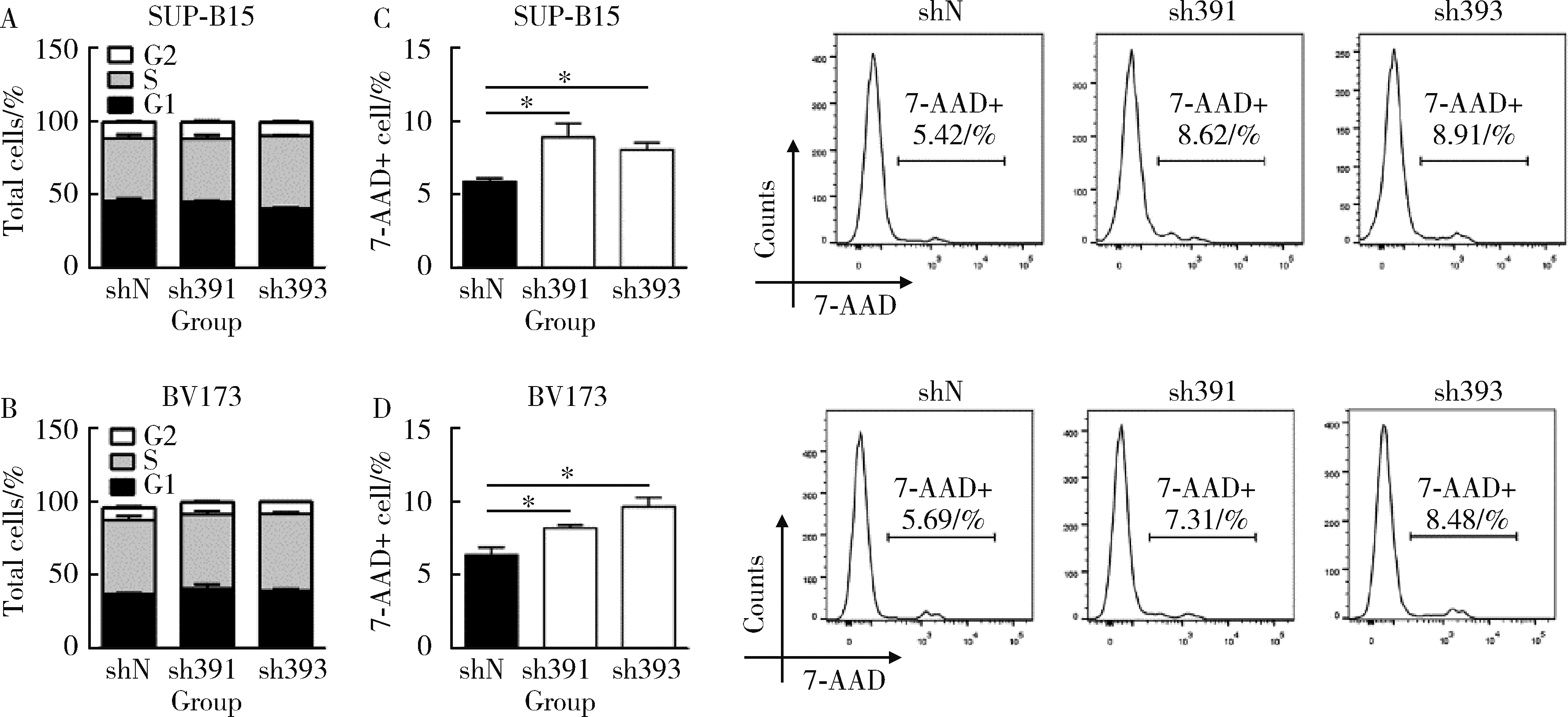
Journal of Peking University (Health Sciences) ›› 2022, Vol. 54 ›› Issue (6): 1238-1243. doi: 10.19723/j.issn.1671-167X.2022.06.031
媛 刘1,2,婉琼 原3,婷 李1,2,平章 王1,2,平 吕1,2,利新 吴4,国瑞 阮4,文玲 韩1,2,晓宁 莫2,*( )
)
CLC Number:
- R733.7
| 1 |
Jain S , Abraham A . BCR-ABL1-like B-acute lymphoblastic leukemia/lymphoma: A comprehensive review[J]. Arch Pathol Lab Med, 2020, 144 (2): 150- 155.
doi: 10.5858/arpa.2019-0194-RA |
| 2 |
Braun TP , Eide CA , Druker BJ . Response and resistance to BCR-ABL1-targeted therapies[J]. Cancer Cell, 2020, 37 (4): 530- 542.
doi: 10.1016/j.ccell.2020.03.006 |
| 3 | Bernt KM , Hunger SP . Current concepts in pediatric Philadelphia chromosome-positive acute lymphoblastic leukemia[J]. Front Oncol, 2014, 4, 54. |
| 4 |
Ultimo S , Simioni C , Martelli AM , et al. PI3K isoform inhibition associated with anti Bcr-Abl drugs shows in vitro increased anti-leukemic activity in Philadelphia chromosome-positive B-acute lymphoblastic leukemia cell lines[J]. Oncotarget, 2017, 8 (14): 23213- 23227.
doi: 10.18632/oncotarget.15542 |
| 5 | Shi R , Lin J , Guo Y , et al. The MEK1/2 inhibitor U0126 reverses imatinib resistance through down-regulating activation of Lyn/ERK signaling pathway in imatinib-resistant K562R leukemia cells[J]. Pharmazie, 2014, 69 (5): 346- 352. |
| 6 |
Tsubaki M . MET/ERK and MET/JNK pathway activation is involved in BCR-ABL inhibitor-resistance in chronic myeloid leukemia[J]. Yakugaku Zasshi, 2018, 138 (12): 1461- 1466.
doi: 10.1248/yakushi.18-00142 |
| 7 |
Kong D , Zhao L , Sun L , et al. MYCN is a novel oncogenic target in adult B-ALL that activates the Wnt/beta-catenin pathway by suppressing DKK3[J]. J Cell Mol Med, 2018, 22 (7): 3627- 3637.
doi: 10.1111/jcmm.13644 |
| 8 |
Janovska P , Bryja V . Wnt signalling pathways in chronic lymphocytic leukaemia and B-cell lymphomas[J]. Br J Pharmacol, 2017, 174 (24): 4701- 4715.
doi: 10.1111/bph.13949 |
| 9 |
Talpaz M , Shah NP , Kantarjian H , et al. Dasatinib in imatinib-resistant Philadelphia chromosome-positive leukemias[J]. N Engl J Med, 2006, 354 (24): 2531- 2541.
doi: 10.1056/NEJMoa055229 |
| 10 |
Druker BJ , Sawyers CL , Kantarjian H , et al. Activity of a specific inhibitor of the BCR-ABL tyrosine kinase in the blast crisis of chronic myeloid leukemia and acute lymphoblastic leukemia with the Philadelphia chromosome[J]. N Engl J Med, 2001, 344 (14): 1038- 1042.
doi: 10.1056/NEJM200104053441402 |
| 11 |
Hong YH , Kim SJ , Kim SK , et al. Impact of imatinib or dasatinib coadministration on in vitro preantral follicle development and oocyte acquisition in cyclophosphamide-treated mice[J]. Clin Exp Reprod Med, 2020, 47 (4): 269- 276.
doi: 10.5653/cerm.2020.03755 |
| 12 | King AC , Pappacena JJ , Tallman MS , et al. Blinatumomab administered concurrently with oral tyrosine kinase inhibitor therapy is a well-tolerated consolidation strategy and eradicates measurable residual disease in adults with Philadelphia chromosome positive acute lymphoblastic leukemia[J]. Leuk Res, 2019, 79 (1): 27- 33. |
| 13 |
Han W , Ding P , Xu M , et al. Identification of eight genes encoding chemokine-like factor superfamily members 1-8 (CKLFSF 1-8) by in silico cloning and experimental validation[J]. Genomics, 2003, 81 (6): 609- 617.
doi: 10.1016/S0888-7543(03)00095-8 |
| 14 |
Amal H , Gong G , Gjoneska E , et al. S-nitrosylation of E3 ubiquitin-protein ligase RNF213 alters non-canonical Wnt/Ca+2 signaling in the P301S mouse model of tauopathy[J]. Transl Psychiatry, 2019, 9 (1): 44.
doi: 10.1038/s41398-019-0388-7 |
| 15 |
Suzuki A , Minamide R , Iwata J . The role of acetyltransferases for the temporal-specific accessibility of beta-catenin to the myogenic gene locus[J]. Sci Rep, 2018, 8 (1): 15057.
doi: 10.1038/s41598-018-32888-z |
| 16 |
Beagle B , Johnson GV . Differential modulation of TCF/LEF-1 activity by the soluble LRP6-ICD[J]. PLoS One, 2010, 5 (7): e11821.
doi: 10.1371/journal.pone.0011821 |
| 17 | Wang K , Yang S , Gao Y , et al. MicroRNA-769-3p inhibits tumor progression in glioma by suppressing ZEB2 and inhibiting the Wnt/beta-catenin signaling pathway[J]. Oncol Lett, 2020, 19 (1): 992- 1000. |
| 18 |
Shao X , Miao M , Qi X , et al. Ras-proximate-1 GTPase-activating protein and Rac2 may play pivotal roles in the initial development of myelodysplastic syndrome[J]. Oncol Lett, 2012, 4 (2): 289- 298.
doi: 10.3892/ol.2012.736 |
| 19 |
Morgenstern Y , Das Adhikari U , Ayyash M , et al. Casein kinase 1-epsilon or 1-delta required for Wnt-mediated intestinal stem cell maintenance[J]. EMBO J, 2017, 36 (20): 3046- 3061.
doi: 10.15252/embj.201696253 |
| 20 |
Gelernter J , Sherva R , Koesterer R , et al. Genome-wide association study of cocaine dependence and related traits: FAM53B identified as a risk gene[J]. Mol Psychiatry, 2014, 19 (6): 717- 723.
doi: 10.1038/mp.2013.99 |
| [1] | Jing LIU,Ai-dong LU,Ying-xi ZUO,Jun WU,Zhi-zhuo HUANG,Yue-ping JIA,Ming-ming DING,Le-ping ZHANG,Jiong QIN. Clinical characteristics and prognosis of seizures in 75 children with acute lymphoblastic leukemia [J]. Journal of Peking University (Health Sciences), 2022, 54(5): 948-953. |
| [2] | Mei-xiang ZHANG,Wen-zhi SHI,Jian-xin LIU,Chun-jian WANG,Yan LI,Wei WANG,Bin JIANG. Clinical characteristics and prognosis of MLL-AF6 positive patients with acute myeloid leukemia [J]. Journal of Peking University (Health Sciences), 2021, 53(5): 915-920. |
| [3] | DUAN Wen-bing, GONG Li-zhong, JIA Jin-song, ZHU Hong-hu, ZHAO Xiao-su, JIANG Qian, ZHAO Ting, WANG Jing, QIN Ya-zhen, HUANG Xiao-jun, JIANG Hao. Clinical features and early treatment effects in intermediate risk and poor risk acute myeloid leukemia with EVI1 positive [J]. Journal of Peking University(Health Sciences), 2017, 49(6): 990-995. |
| Viewed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
Full text 156
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
|
Abstract 335
|
|
|||||||||||||||||||||||||||||||||||||||||||||||||
Cited |
|
|||||||||||||||||||||||||||||||||||||||||||||||||
| Shared | ||||||||||||||||||||||||||||||||||||||||||||||||||
| Discussed | ||||||||||||||||||||||||||||||||||||||||||||||||||
|
||