Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (5): 1005-1009. doi: 10.19723/j.issn.1671-167X.2025.05.029
Previous Articles Next Articles
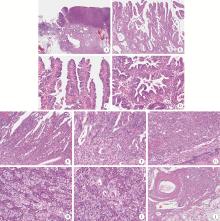
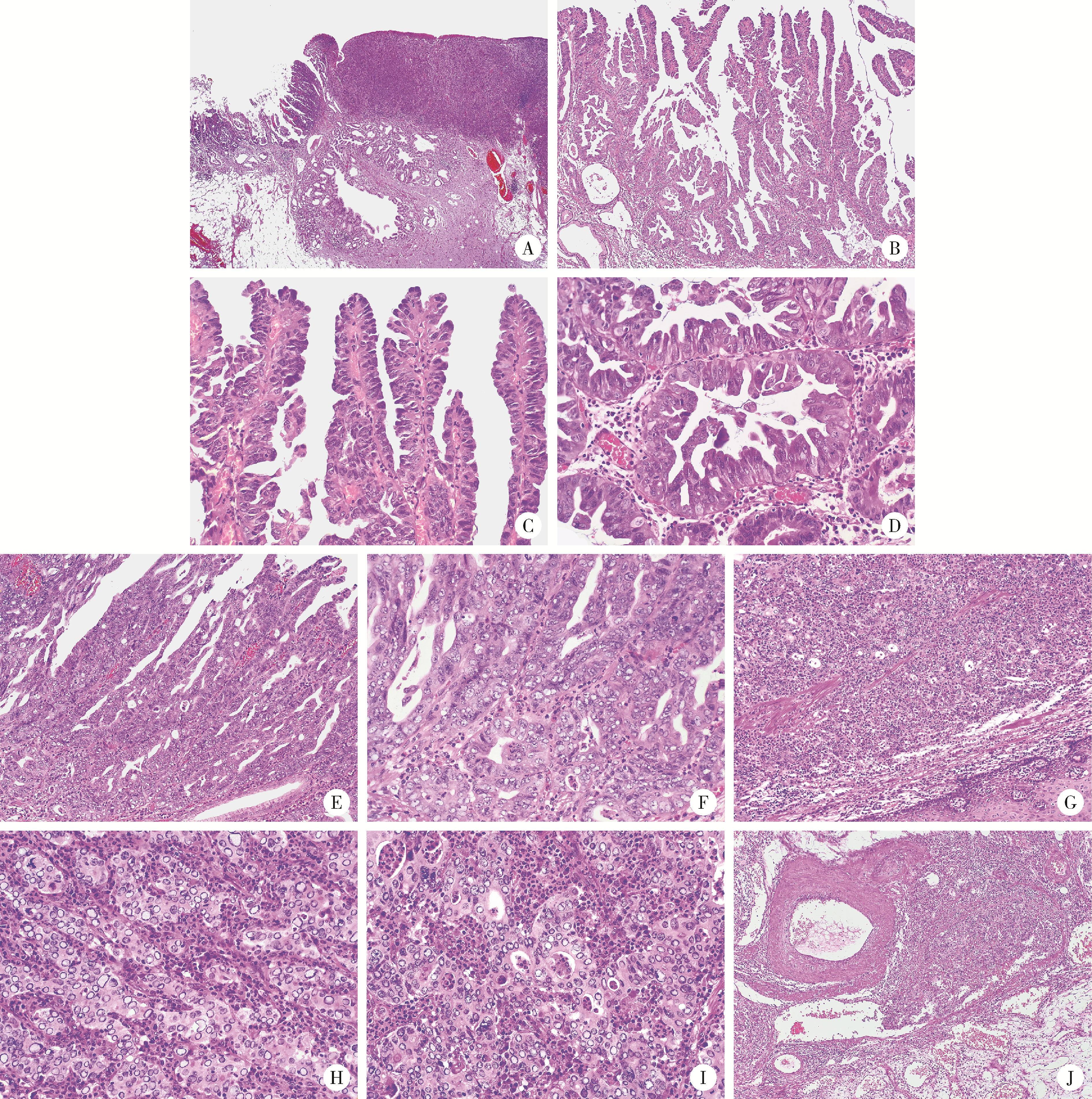
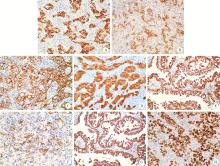
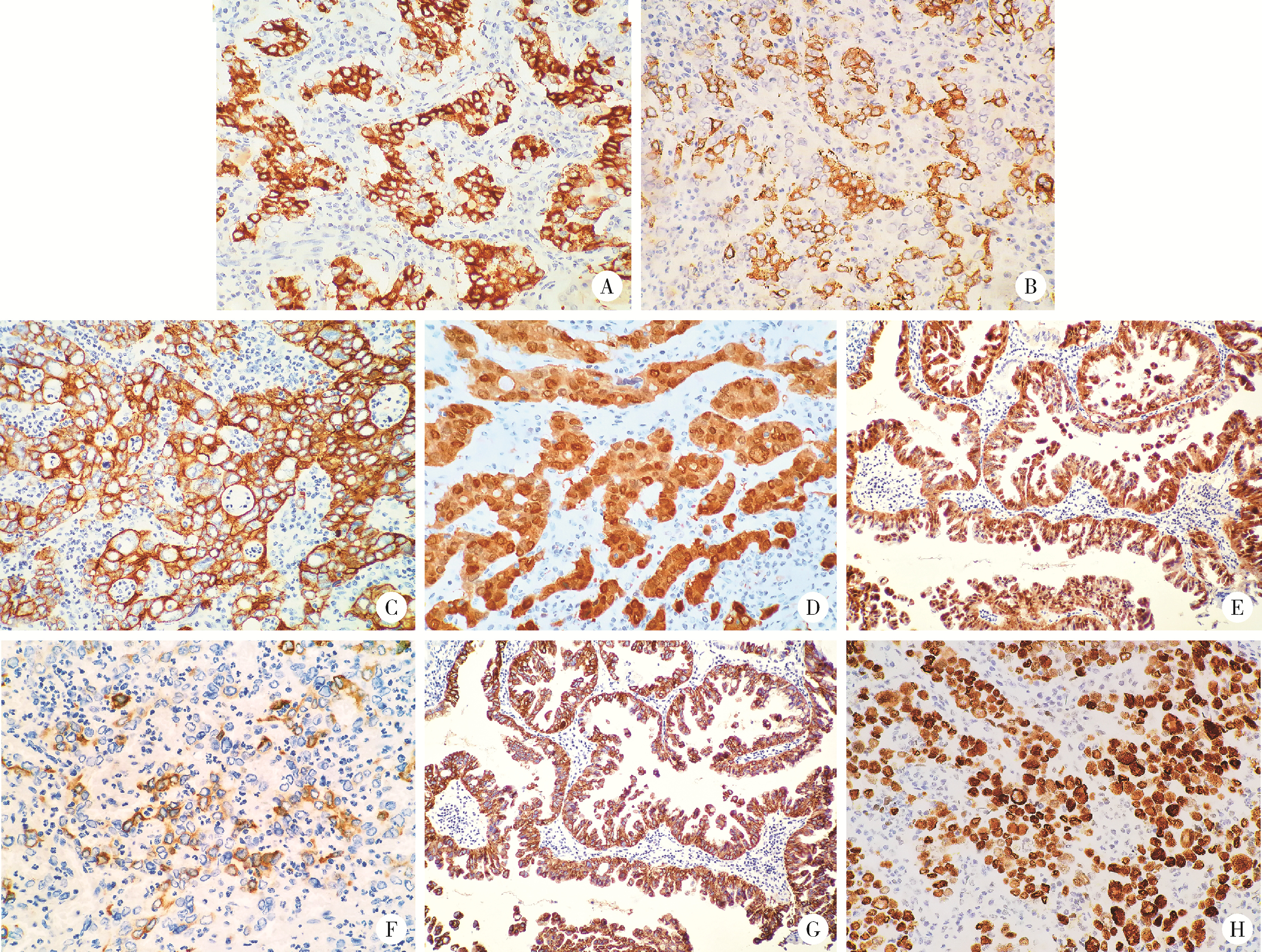
Neuroendocrine carcinoma with significantly vacuolar nucleus at the esophagogastric junction: A case report
Weihua HOU1, Shujie SONG2, Zhongyue SHI3, Lu LIU2, Mulan JIN3,*( )
)
- 1. Department of Pathology, Xiangcheng County People's Hospital, Xuchang 461700, Henan, China
2. Department of Gastroenterology, 989 Hospital of Joint Logistic Support Force, Pingdingshan 467099, Henan, China
3. Department of Pathology, Beijing Chaoyang Hospital, Capital Medical University, Beijing 100020, China
CLC Number:
- R735.2
| 1 |
doi: 10.32074/1591-951X-227 |
| 2 |
Klimstra DS, Kloppel G, La Rosa S, et al. Classification of neuroendocrine neoplasms of the digestive system[M]//WHO classification of tumours editorial board. WHO classification of tumours, digestive system tumours. 5th ed. Lyon, France: IARC Press, 2019: 16-19.
|
| 3 |
doi: 10.1007/s11864-022-00968-y |
| 4 |
doi: 10.1007/s12022-016-9432-9 |
| 5 |
doi: 10.3390/jcm9010273 |
| 6 |
doi: 10.3748/wjg.v28.i19.2076 |
| 7 |
doi: 10.3748/wjg.v28.i8.794 |
| 8 |
Lam AK, Kumarasinghe MP. Adenocarcinoma of the oesophagus and oesophagus and oesophagogastric junction NOS[M]//WHO classification of tumours editorial board. WHO classification of tumours, digestive system tumours. 5th ed. Lyon, France: IARC Press, 2019: 38-43.
|
| 9 |
|
| 10 |
doi: 10.1016/j.humpath.2020.12.008 |
| 11 |
doi: 10.1007/s12022-021-09676-z |
| 12 |
|
| 13 |
doi: 10.1097/PAI.0000000000000906 |
| [1] | Kun WANG,Zhi-jie XU,Ying GE,Zhi-wei XIA,Li-ping DUAN. Study of etiology and esophageal motility characteristics of esophagogastric junction outlet obstruction patients [J]. Journal of Peking University (Health Sciences), 2020, 52(5): 828-835. |
|
||