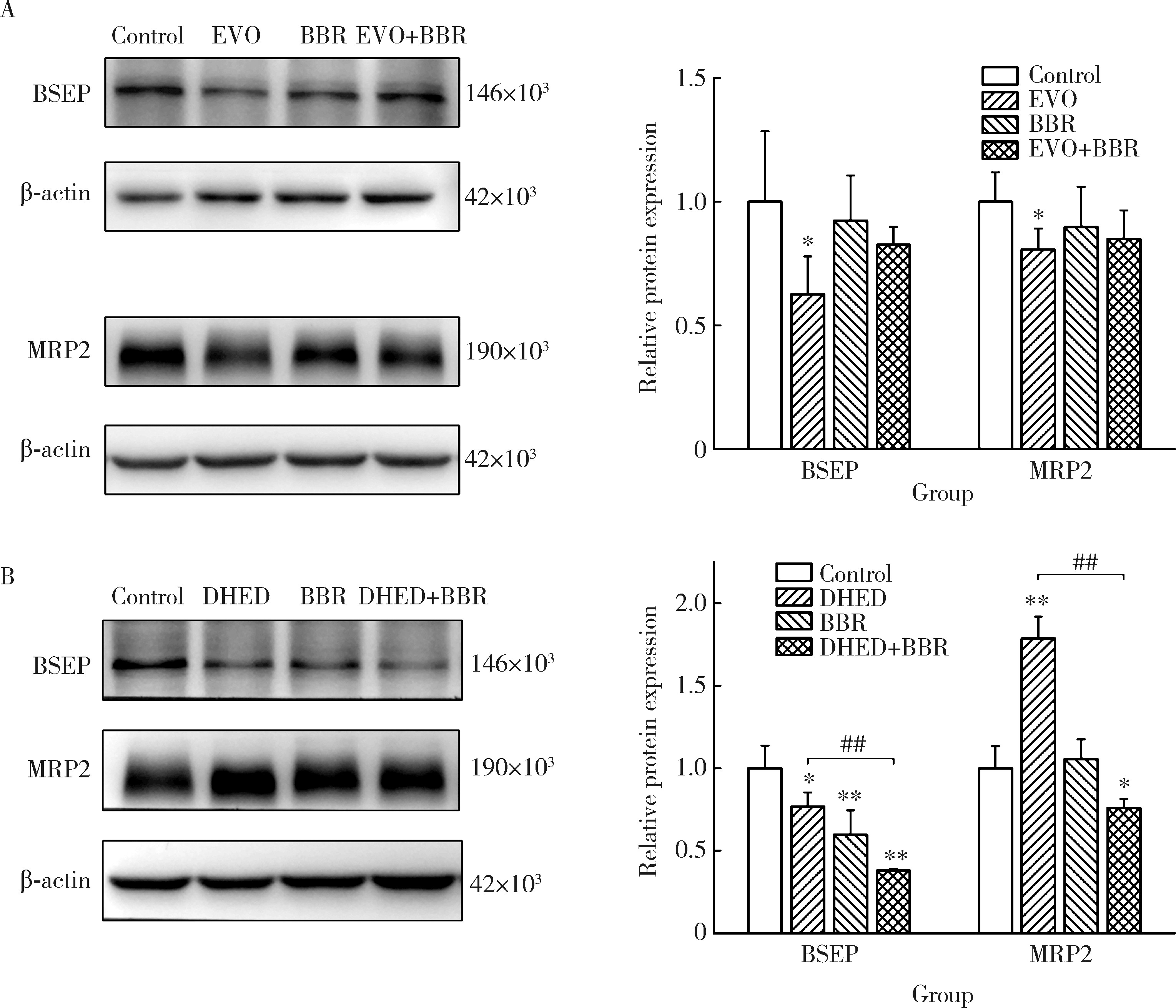
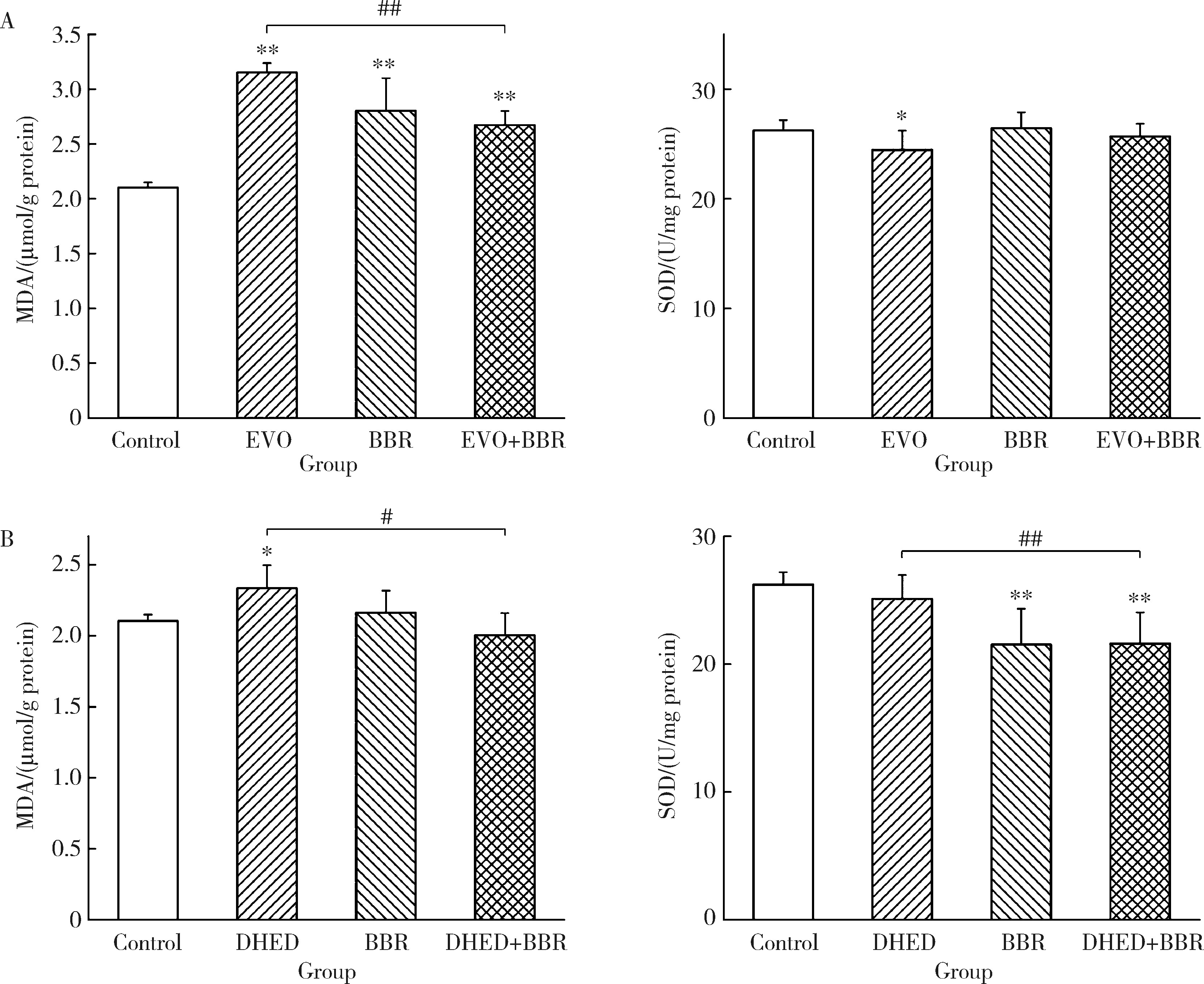
Journal of Peking University (Health Sciences) ›› 2025, Vol. 57 ›› Issue (5): 926-933. doi: 10.19723/j.issn.1671-167X.2025.05.017
Previous Articles Next Articles
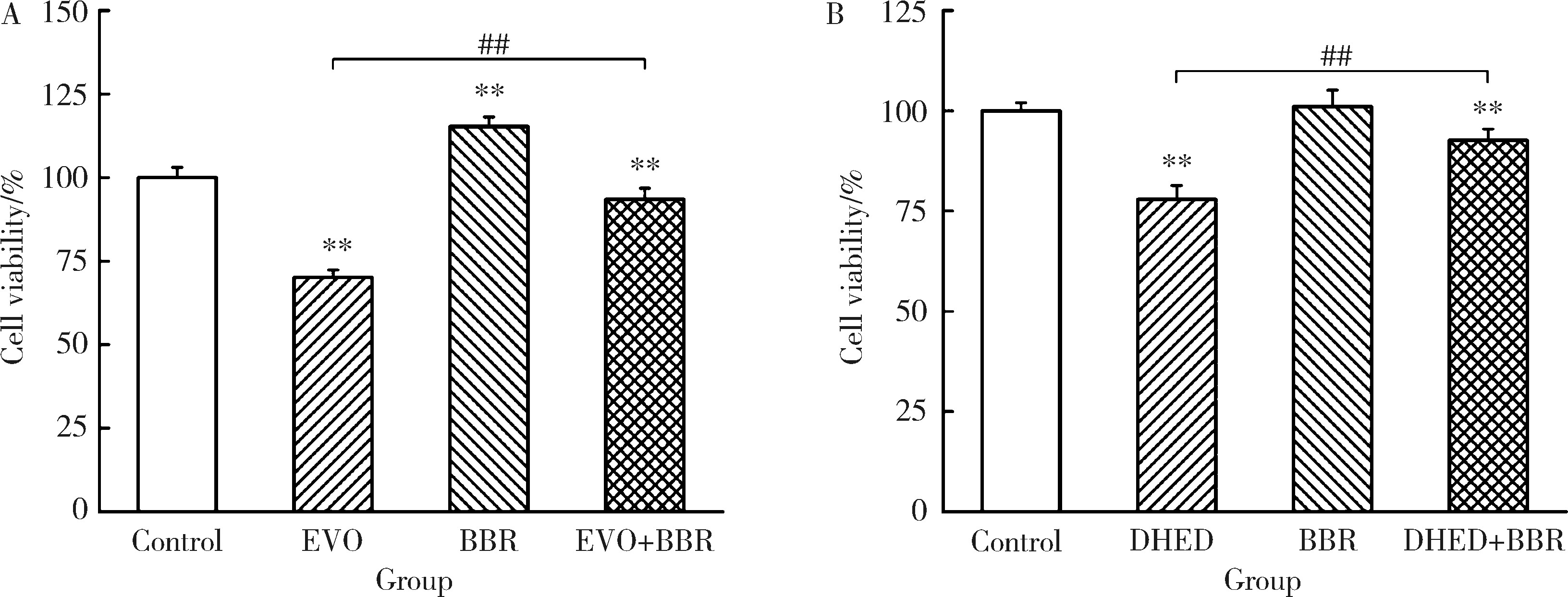
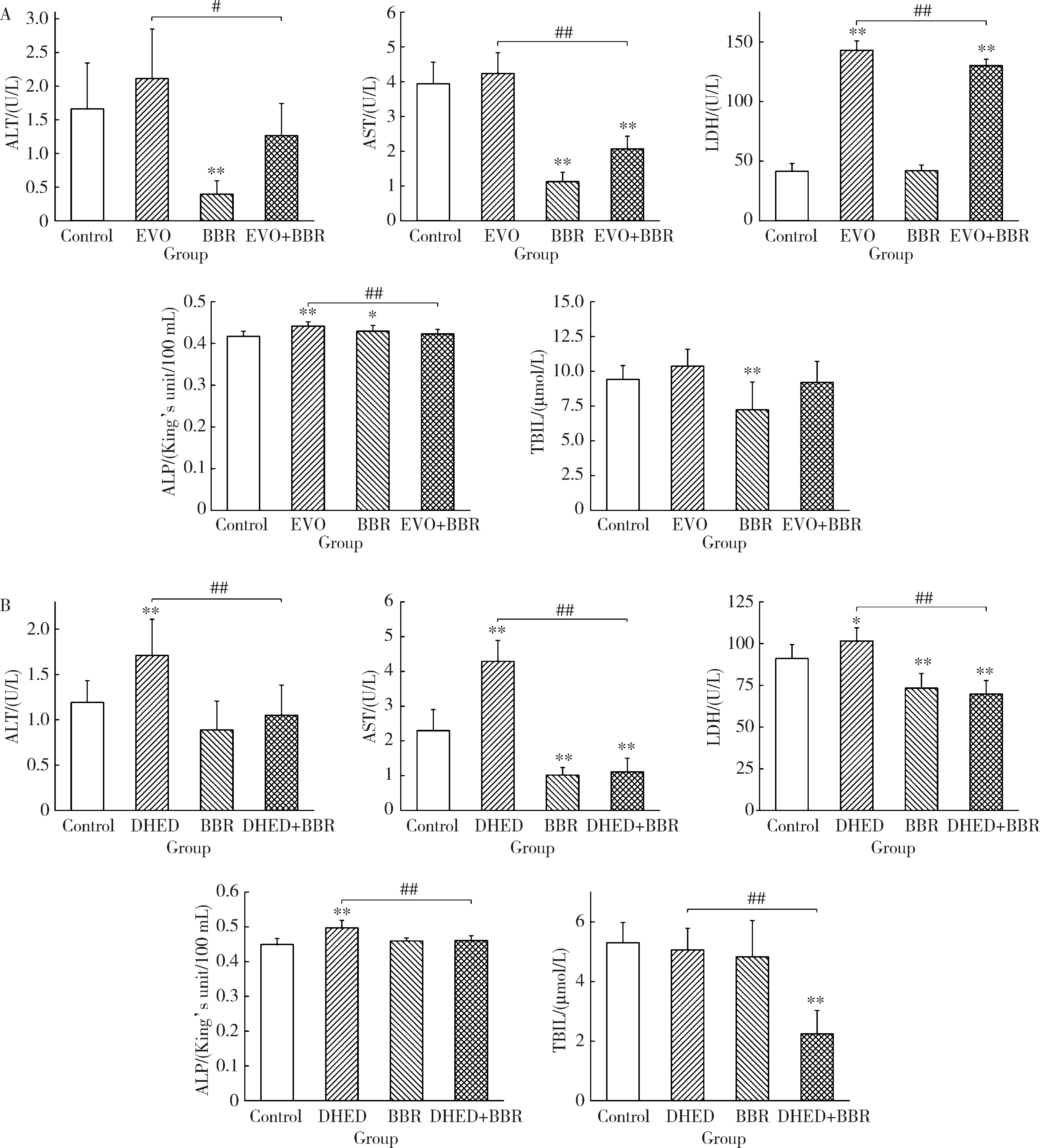
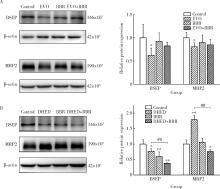
Effect of the combination of alkaloids from Euodiae Fructus and berberine in Zuojin Pill on cytotoxicity in HepG2 cells
Yadong GAO1,2, An ZHU1,3, Ludi LI1, Yingzi LI1, Qi WANG1,4,5,*( )
)
- 1. Department of Toxicology, Peking University School of Public Health, Beijing 100191, China
2. Fujian Provincial Key Laboratory of Zoonosis Research, Fujian Provincial Center for Disease Control and Prevention, Fuzhou 350012, China
3. Key Laboratory of Ministry of Education for Gastrointestinal Cancer, School of Basic Medical Sciences, Fujian Medical University, Fuzhou 350108, China
4. Key Laboratory of State Administration of Traditional Chinese Medicine for Compatibility Toxicology, Beijing 100191, China
5. Beijing Key Laboratory of Toxicological Research and Risk Assessment for Food Safety, Beijing 100191, China
CLC Number:
- R114
| 1 |
国家药典委员会. 中华人民共和国药典(一部2020年版)[M]. 北京: 中国医药科技出版社, 2020: 178.
|
| 2 |
黄伟, 李晓骄阳, 孙蓉. 吴茱萸水提组分多次给药对小鼠肝毒性的"量-时-毒"关系研究[J]. 中国中药杂志, 2012, 37(15): 2223- 2227.
|
| 3 |
刘颖, 杨润芳, 夏祺悦, 等. 吴茱萸醇提物重复给药的靶器官毒性研究[J]. 现代预防医学, 2015, 42(14): 2600- 2603.
|
| 4 |
彭成. 试论中药配伍研究的方法与实践[J]. 中药与临床, 2012, 3(1): 1- 4.
|
| 5 |
doi: 10.1016/j.ejphar.2020.173655 |
| 6 |
doi: 10.3390/molecules22020214 |
| 7 |
doi: 10.1124/pr.58.3.10 |
| 8 |
彭求贤, 蔡红兵, 史珏, 等. 黄连配伍吴茱萸后生物碱类成分的含量变化[J]. 中药材, 2012, 35(5): 742- 744.
|
| 9 |
倪建新, 林跃虹, 陈妙珠. 左金丸配伍意义的药物代谢动力学分析[J]. 中国当代医药, 2012, 19(5): 19- 20.
|
| 10 |
梁瑞峰, 张峰, 刘方洲, 等. 吴茱萸对黄连中小檗碱组织分布及肝脏摄取的影响[J]. 中医研究, 2014, 27(1): 64- 66.
|
| 11 |
黄果, 李凯鹏, 杨洁, 等. 黄连与吴茱萸合用大鼠体外肝代谢研究[J]. 中国中药杂志, 2010, 35(5): 651- 653.
|
| 12 |
doi: 10.1016/j.phymed.2008.05.002 |
| 13 |
doi: 10.1186/s12944-017-0628-x |
| 14 |
高亚东, 朱安, 李璐迪, 等. 吴茱萸碱对HepG2细胞毒性及其机制[J]. 北京大学学报(医学版), 2021, 53(6): 1107- 1114.
doi: 10.19723/j.issn.1671-167X.2021.06.017 |
| 15 |
|
| 16 |
|
| 17 |
doi: 10.1016/j.hepres.2006.01.009 |
| 18 |
doi: 10.3748/wjg.15.1677 |
| 19 |
doi: 10.1016/j.jhep.2005.03.017 |
| 20 |
doi: 10.1038/clpt.2014.158 |
| 21 |
doi: 10.1067/mcp.2001.114667 |
| 22 |
|
| 23 |
doi: 10.1186/s12906-016-1367-7 |
| 24 |
doi: 10.1074/jbc.M116.757567 |
| 25 |
doi: 10.1124/dmd.118.083691 |
| 26 |
doi: 10.1016/j.redox.2013.07.006 |
| 27 |
doi: 10.1038/35030140 |
| 28 |
doi: 10.1080/13510002.2016.1140406 |
| 29 |
doi: 10.1007/s11356-019-07232-1 |
| [1] | GAO Ya-dong,ZHU An,LI Lu-di,ZHANG Tao,WANG Shuo,SHAN Dan-ping,LI Ying-zi,WANG Qi. Cytotoxicity and underlying mechanism of evodiamine in HepG2 cells [J]. Journal of Peking University (Health Sciences), 2021, 53(6): 1107-1114. |
|
||